Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes
- PMID: 20148561
- PMCID: PMC2836128
- DOI: 10.1021/ja910989n
Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes
Abstract
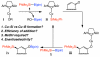
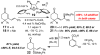
An efficient Cu-catalyzed protocol for enantioselective addition of a dimethylphenylsilanyl group to a wide range of cyclic and acyclic unsaturated ketones, esters, acrylonitriles, and alpha,beta,gamma,delta-dienones is disclosed. Reactions are performed in the presence of 1-2 mol % of commercially available and inexpensive CuCl, a readily accessible monodentate imidazolinium salt, and commercially available (dimethylphenylsilyl)pinacolatoboron. Cu-catalyzed enantioselective conjugate additions proceed to completion within only 2 h to afford the desired silanes in 87-97% yield and 90:10-99:1 enantiomeric ratio (er). Use of a proton source (e.g., MeOH) is not required; accordingly, synthetically versatile alpha-silyl boron enolates can be obtained. The special utility of the present protocol, in comparison with the related catalytic enantioselective aldol and boronate conjugate additions, is discussed and illustrated through various functionalizations of the enantiomerically enriched beta-silylcarbonyls.
Figures
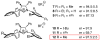
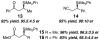

References
-
-
For reviews on the use of organosilanes in organic synthesis, see: Chan TH, Wang D. Chem. Rev. 1992;92:995. Jones GR, Landais Y. Tetrahedron. 1996;52:7599. Fleming I, Barbero A, Walter D. Chem. Rev. 1997;97:2063. Suginome M, Ito Y. Chem. Rev. 2000;100:3221.
-
-
-
For a review on the utility of β-silylcarbonyls in synthesis, see: Fleming I. Science of Synthesis. Vol. 4. Thieme; Stuttgart, Germany: 2002. p. 927.
-
-
-
Enantiomerically enriched β-silylcarbonyls have been prepared by catalytic conjugate hydride additions to trisubstituted Si-substituted enones. See: Lipshutz BH, Tanaka N, Taft BR, Lee C.-t. Org. Lett. 2006;8:1963.
-
-
-
Enantiomerically enriched β-silylcarbonyls can be accessed by catalytic conjugate additions of alkyl or aryl groups to silyl-substituted enones. See: Shintani R, Okamoto K, Hayashi T. Org. Lett. 2005;7:4757. Balskus EP, Jacobsen EN. J. Am. Chem. Soc. 2006;128:6810. Kacprzynski MA, Kazane SA, May TL, Hoveyda AH. Org. Lett. 2007;9:3187.
-
-
-
Catalytic non-enantioselective methods that afford β-silylcarbonyls have been disclosed. For example, see: Conjugate silane additions: Lipshutz BH, Sclafani JA, Takanami T. J. Am. Chem. Soc. 1998;120:4021. Auer G, Weiner B, Oestreich M. Synthesis. 2006:2113. Conjugate disilane additions: Tamao K, Okazaki S, Kumada M. J. Organomet. Chem. 1978;146:87. Ito H, Ishizuka T, Tateiwa J.-i., Sonoda M, Hosomi A. J. Am. Chem. Soc. 1998;120:11196. Ogoshi S, Tomiyasu S, Morita M, Kurosawa H. J. Am. Chem. Soc. 2002;124:11598. Clark CT, Lake JF, Scheidt KA. J. Am. Chem. Soc. 2004;126:84.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

