The biogenesis of chylomicrons
- PMID: 20148678
- PMCID: PMC4861230
- DOI: 10.1146/annurev-physiol-021909-135801
The biogenesis of chylomicrons
Abstract
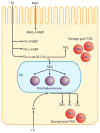
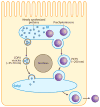
The absorption of dietary fat is of increasing concern given the rise of obesity not only in the United States but throughout the developed world. This review explores what happens to dietary fat within the enterocyte. Absorbed fatty acids and monoacylglycerols are required to be bound to intracellular proteins and/or to be rapidly converted to triacylglycerols to prevent cellular membrane disruption. The triacylglycerol produced at the level of the endoplasmic reticulum (ER) is either incorporated into prechylomicrons within the ER lumen or shunted to triacylglycerol storage pools. The prechylomicrons exit the ER in a specialized transport vesicle in the rate-limiting step in the intracellular transit of triacylglycerol across the enterocyte. The prechylomicrons are further processed in the Golgi and are transported to the basolateral membrane via a separate vesicular system for exocytosis into the intestinal lamina propria. Fatty acids and monoacylglycerols entering the enterocyte via the basolateral membrane are also incorporated into triacylglycerol, but the basolaterally entering lipid is much more likely to enter the triacylglycerol storage pool than the lipid entering via the apical membrane.
Figures
References
-
- Stralfors P. Autolysis of isolated adipocytes by endogenously produced fatty acids. FEBS Lett. 1990;263:153–54. - PubMed
-
- Bass NA, Manning JA, Ockner RK, Gordon JI, Seetharam S, Alpers DH. Regulation of the biosynthesis of two distinct fatty acid–binding proteins in rat liver and intestine. J Biol Chem. 1985;260:1432–36. - PubMed
-
- Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, et al. Intestinal-type and liver-type fatty acid–binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–35. - PubMed
-
- Shields HM, Bates ML, Bass NM, Best CJ, Alpers DH, Ockner RK. Light microscopic immunocytochemical localization of hepatic and intestinal types of fatty acid–binding proteins in rat small intestine. J Lipid Res. 1986;27:549–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

