Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning
- PMID: 20148810
- PMCID: PMC2856406
- DOI: 10.1111/j.1600-6143.2010.03018.x
Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning
Abstract
Establishment of mixed chimerism through transplantation of allogeneic donor bone marrow (BM) into sufficiently conditioned recipients is an effective experimental approach for the induction of transplantation tolerance. Clinical translation, however, is impeded by the lack of feasible protocols devoid of cytoreductive conditioning (i.e. irradiation and cytotoxic drugs/mAbs). The therapeutic application of regulatory T cells (Tregs) prolongs allograft survival in experimental models, but appears insufficient to induce robust tolerance on its own. We thus investigated whether mixed chimerism and tolerance could be realized without the need for cytoreductive treatment by combining Treg therapy with BM transplantation (BMT). Polyclonal recipient Tregs were cotransplanted with a moderate dose of fully mismatched allogeneic donor BM into recipients conditioned solely with short-course costimulation blockade and rapamycin. This combination treatment led to long-term multilineage chimerism and donor-specific skin graft tolerance. Chimeras also developed humoral and in vitro tolerance. Both deletional and nondeletional mechanisms contributed to maintenance of tolerance. All tested populations of polyclonal Tregs (FoxP3-transduced Tregs, natural Tregs and TGF-beta induced Tregs) were effective in this setting. Thus, Treg therapy achieves mixed chimerism and tolerance without cytoreductive recipient treatment, thereby eliminating a major toxic element impeding clinical translation of this approach.
Figures
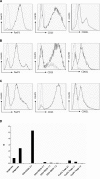

 , 4 × 106, n = 7) or without (◊, n = 6) recipient-derived FoxP3-Tregs, (B) with (
, 4 × 106, n = 7) or without (◊, n = 6) recipient-derived FoxP3-Tregs, (B) with ( , 3 × 106, n = 4) or without (◊, n = 7) recipient-derived nTregs or (C) with (
, 3 × 106, n = 4) or without (◊, n = 7) recipient-derived nTregs or (C) with ( , 5 × 106, n = 6) or without (◊, n = 7) recipient-derived iTregs, respectively. Only recipients treated with any of the Treg populations developed chimerism. Donor (H-2Dd+) chimerism among leukocytes of the myeloid (Mac1+) lineage was assessed by flow cytometry of peripheral blood at multiple time points and is shown as mean percent (error bars indicate standard deviation; nTreg and iTreg groups were done in the same experiment, therefore the control group is shown twice in B + C). Repeat experiments performed with nTregs and iTregs showed similar results. **p < 0.005, *p < 0.05 with versus without Tregs (two-sided Student's t-test). (D) B6 mice received 2 × 107 Balb/c BM cells under the cover of costimulation blockade and 5 × 106 iTregs with or without rapamycin. Recipients treated without rapamycin failed to develop chimerism (• iTregs with rapamycin n = 8;
, 5 × 106, n = 6) or without (◊, n = 7) recipient-derived iTregs, respectively. Only recipients treated with any of the Treg populations developed chimerism. Donor (H-2Dd+) chimerism among leukocytes of the myeloid (Mac1+) lineage was assessed by flow cytometry of peripheral blood at multiple time points and is shown as mean percent (error bars indicate standard deviation; nTreg and iTreg groups were done in the same experiment, therefore the control group is shown twice in B + C). Repeat experiments performed with nTregs and iTregs showed similar results. **p < 0.005, *p < 0.05 with versus without Tregs (two-sided Student's t-test). (D) B6 mice received 2 × 107 Balb/c BM cells under the cover of costimulation blockade and 5 × 106 iTregs with or without rapamycin. Recipients treated without rapamycin failed to develop chimerism (• iTregs with rapamycin n = 8;  iTregs without rapamycin, n = 8; p = 0.0002, Fisher's exact test). Donor (H-2Dd+) chimerism among leukocytes of the myeloid (Mac1+) lineage is shown 2 weeks post-BMT as scatter plot (mean and standard deviation are indicated).
iTregs without rapamycin, n = 8; p = 0.0002, Fisher's exact test). Donor (H-2Dd+) chimerism among leukocytes of the myeloid (Mac1+) lineage is shown 2 weeks post-BMT as scatter plot (mean and standard deviation are indicated).



Similar articles
-
Therapeutic efficacy of polyclonal tregs does not require rapamycin in a low-dose irradiation bone marrow transplantation model.Transplantation. 2011 Aug 15;92(3):280-8. doi: 10.1097/TP.0b013e3182241133. Transplantation. 2011. PMID: 21697774
-
T-regulatory cell treatment prevents chronic rejection of heart allografts in a murine mixed chimerism model.J Heart Lung Transplant. 2014 Apr;33(4):429-37. doi: 10.1016/j.healun.2013.11.004. Epub 2013 Nov 28. J Heart Lung Transplant. 2014. PMID: 24468120 Free PMC article.
-
Bone marrow transplantation combined with mesenchymal stem cells induces immune tolerance without cytotoxic conditioning.J Surg Res. 2011 Nov;171(1):e123-31. doi: 10.1016/j.jss.2011.06.020. Epub 2011 Jul 13. J Surg Res. 2011. PMID: 21920556
-
Deletional and regulatory mechanisms coalesce to drive transplantation tolerance through mixed chimerism.Eur J Immunol. 2015 Sep;45(9):2470-9. doi: 10.1002/eji.201545494. Eur J Immunol. 2015. PMID: 26200095 Review.
-
Mechanistic and therapeutic role of regulatory T cells in tolerance through mixed chimerism.Curr Opin Organ Transplant. 2010 Dec;15(6):725-30. doi: 10.1097/MOT.0b013e3283401755. Curr Opin Organ Transplant. 2010. PMID: 20881493 Review.
Cited by
-
CTLA4Ig Improves Murine iTreg Induction via TGFβ and Suppressor Function In Vitro.J Immunol Res. 2018 Jul 2;2018:2484825. doi: 10.1155/2018/2484825. eCollection 2018. J Immunol Res. 2018. PMID: 30057914 Free PMC article.
-
Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques.Transplantation. 2017 Feb;101(2):274-283. doi: 10.1097/TP.0000000000001559. Transplantation. 2017. PMID: 27846155 Free PMC article.
-
Differential outcomes in prediabetic vs. overtly diabetic NOD mice nonmyeloablatively conditioned with costimulatory blockade.Exp Hematol. 2011 Oct;39(10):977-85. doi: 10.1016/j.exphem.2011.06.008. Epub 2011 Jul 1. Exp Hematol. 2011. PMID: 21726515 Free PMC article.
-
Transplantation tolerance: Clinical potential of regulatory T cells.Self Nonself. 2011 Jan;2(1):26-34. doi: 10.4161/self.2.1.15422. Epub 2011 Jan 1. Self Nonself. 2011. PMID: 21776332 Free PMC article.
-
Report from IPITA-TTS Opinion Leaders Meeting on the Future of β-Cell Replacement.Transplantation. 2016 Feb;100 Suppl 2(Suppl 2):S1-44. doi: 10.1097/TP.0000000000001055. Transplantation. 2016. PMID: 26840096 Free PMC article. No abstract available.
References
-
- Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–370. - PubMed
-
- Sykes M, Szot GL, Swenson K, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nat Med. 1997;3:783–787. - PubMed
-
- Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. - PubMed
-
- Shirasugi N, Adams AB, Durham MM, et al. Prevention of chronic rejection in murine cardiac allografts: A comparison of chimerism- and nonchimerism-inducing costimulation blockade-based tolerance induction regimens. J Immunol. 2002;169:2677–2684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

