Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants
- PMID: 20148890
- PMCID: PMC2839267
- DOI: 10.1111/j.1476-5381.2009.00633.x
Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants
Abstract
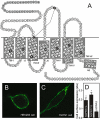
Background and purpose: In humans and non-human primates, the 7TM receptor GPR17 exists in two isoforms differing only by the length of the N-terminus. Of these, only the short isoform has previously been characterized. Hence, we investigated gene expression and ligand-binding profiles of both splice variants and furthermore uncovered and characterized constitutive activity of both isoforms.
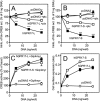
Experimental approach: Expression levels of the hGPR17 isoforms were determined in several brain regions as well as heart and kidney using quantitative RT-PCR. A CREB reporter assay and [(35)S]-GTPgammaS binding were employed to assess the constitutive activity and the activation by UDP, UDP-glucose and -galactose and the cysteinyl leukotrienes LTC(4) and LTD(4). Leukotriene binding and induction of internalization were furthermore tested using homologous competition binding and antibody-feeding experiments respectively.
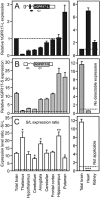
Key results: The short isoform (hGPR17-S) was expressed more abundantly (eight- to 23-fold) in the brain than the long isoform (hGPR17-L), whereas the opposite was observed in heart and kidney. As previously reported, the uracil nucleotides activated hGPR17-S with micromolar potencies. However, much lower potencies were observed for hGPR17-L with a 50- to 170-fold increase in EC(50). Furthermore, contrary to previous reports, neither of the isoforms was activated or bound by the cysteinyl leukotrienes. Finally, both receptors were demonstrated to be constitutively active through Galpha(i).
Conclusions and implications: We present the first isoform-specific characterization of GPR17 and show that differences exist between the isoforms, in both expression pattern and pharmacological profile. In turn, our results indicate that the two human isoforms might serve tissue-specific functions.
Figures





References
-
- Benned-Jensen T, Rosenkilde MM. Structural motifs of importance for the constitutive activity of the orphan 7TM receptor EBI2: analysis of receptor activation in the absence of an agonist. Mol Pharmacol. 2008;74:1008–1021. - PubMed
-
- Blasius R, Weber RG, Lichter P, Ogilvie A. A novel orphan G protein-coupled receptor primarily expressed in the brain is localized on human chromosomal band 2q21. J Neurochem. 1998;70:1357–1365. - PubMed
-
- Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, et al. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132:2206–2218. - PubMed
-
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

