The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase
- PMID: 20148966
- PMCID: PMC2874831
- DOI: 10.1111/j.1742-4658.2010.07588.x
The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase
Abstract
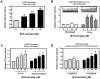
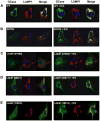
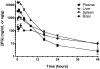
Gaucher disease is caused by mutations in the gene that encodes the lysosomal enzyme acid beta-glucosidase (GCase). We have shown previously that the small molecule pharmacological chaperone isofagomine (IFG) binds and stabilizes N370S GCase, resulting in increased lysosomal trafficking and cellular activity. In this study, we investigated the effect of IFG on L444P GCase. Incubation of Gaucher patient-derived lymphoblastoid cell lines (LCLs) or fibroblasts with IFG led to approximately 3.5- and 1.3-fold increases in L444P GCase activity, respectively, as measured in cell lysates. The effect in fibroblasts was increased approximately 2-fold using glycoprotein-enrichment, GCase-immunocapture, or by incubating cells overnight in IFG-free media prior to assay, methods designed to maximize GCase activity by reducing IFG carryover and inhibition in the enzymatic assay. IFG incubation also increased the lysosomal trafficking and in situ activity of L444P GCase in intact cells, as measured by reduction in endogenous glucosylceramide levels. Importantly, this reduction was seen only following three-day incubation in IFG-free media, underscoring the importance of IFG removal to restore lysosomal GCase activity. In mice expressing murine L444P GCase, oral administration of IFG resulted in significant increases (2- to 5-fold) in GCase activity in disease-relevant tissues, including brain. Additionally, eight-week IFG administration significantly lowered plasma chitin III and IgG levels, and 24-week administration significantly reduced spleen and liver weights. Taken together, these data suggest that IFG can increase the lysosomal activity of L444P GCase in cells and tissues. Moreover, IFG is orally available and distributes into multiple tissues, including brain, and may thus merit therapeutic evaluation for patients with neuronopathic and non-neuronopathic Gaucher disease.
Figures




References
-
- Beutler E, Grabowski G. In: The Metabolic and Molecular Bases of Inherited Disease 2006. Scriver C, Beaudet A, Sly W, Valle D, editors. McGraw-Hill; New York: 2001.
-
- Butters TD. Gaucher disease. Curr Opin Chem Biol. 2007;11:412–418. - PubMed
-
- Beutler E, Gelbart T, West C. Identification of six new Gaucher disease mutations. Genomics. 1993;15:203–205. - PubMed
-
- Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet Test. 1997;1:5–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

