Two sources of endogenous hydrogen peroxide in Escherichia coli
- PMID: 20149100
- PMCID: PMC3049997
- DOI: 10.1111/j.1365-2958.2010.07059.x
Two sources of endogenous hydrogen peroxide in Escherichia coli
Abstract
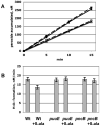
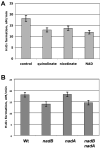
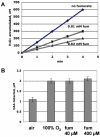
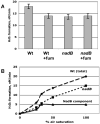
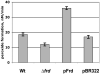
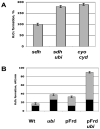
Mechanisms of hydrogen peroxide generation in Escherichia coli were investigated using a strain lacking scavenging enzymes. Surprisingly, the deletion of many abundant flavoenzymes that are known to autoxidize in vitro did not substantially lessen overall H(2)O(2) formation. However, H(2)O(2) production diminished by 25-30% when NadB turnover was eliminated. The flavin-dependent desaturating dehydrogenase, NadB uses fumarate as an electron acceptor in anaerobic cells. Experiments showed that aerobic NadB turnover depends upon its oxidation by molecular oxygen, with H(2)O(2) as a product. This reaction appears to be mechanistically adventitious. In contrast, most desaturating dehydrogenases are associated with the respiratory chain and deliver electrons to fumarate anaerobically or oxygen aerobically without the formation of toxic by-products. Presumably, NadB can persist as an H(2)O(2)-generating enzyme because its flux is limited. The anaerobic respiratory enzyme fumarate reductase uses a flavoprotein subunit that is homologous to NadB and accordingly forms substantial H(2)O(2) upon aeration. This tendency is substantially suppressed by cytochrome oxidase. Thus cytochrome d oxidase, which is prevalent among anaerobes, may diminish intracellular H(2)O(2) formation by the anaerobic respiratory chain, whenever these organisms encounter oxygen. These two examples reveal biochemical and physiological arrangements through which evolution has minimized the rate of intracellular oxidant formation.
Figures
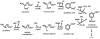
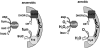
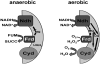
References
-
- Blaut M, Whittaker K, Valdovinos A, Ackrell BAC, Gunsalus RP, Cecchini G. Fumarate reductase mutants of Escherichia coli that lack covalently bound flavin. J Biol Chem. 1989;264:13599–13604. - PubMed
-
- Bolivar F, Rodriguez RL, Green PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of plasmid pMB9. Gene. 1977;2:95–113. - PubMed
-
- Bunch PK, Mat-Jan F, Lee N, Clark DP. The ldhA gene encoding fermentative lactate dehydrogenase of Escherichia coli. Microbiology. 1997;143:187–195. - PubMed
-
- D’mello R, Hill S, Poole RK. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

