The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals
- PMID: 20149227
- PMCID: PMC2829555
- DOI: 10.1186/1471-2148-10-38
The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals
Abstract
Background: Aquaporins are integral membrane proteins that facilitate the transport of water and small solutes across cell membranes. These proteins are vital for maintaining water homeostasis in living organisms. In mammals, thirteen aquaporins (AQP0-12) have been characterized, but in lower vertebrates, such as fish, the diversity, structure and substrate specificity of these membrane channel proteins are largely unknown.
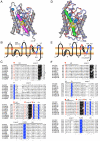
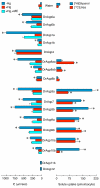
Results: The screening and isolation of transcripts from the zebrafish (Danio rerio) genome revealed eighteen sequences structurally related to the four subfamilies of tetrapod aquaporins, i.e., aquaporins (AQP0, -1 and -4), water and glycerol transporters or aquaglyceroporins (Glps; AQP3 and AQP7-10), a water and urea transporter (AQP8), and two unorthodox aquaporins (AQP11 and -12). Phylogenetic analyses of nucleotide and deduced amino acid sequences demonstrated dual paralogy between teleost and human aquaporins. Three of the duplicated zebrafish isoforms have unlinked loci, two have linked loci, while DrAqp8 was found in triplicate across two chromosomes. Genomic sequencing, structural analysis, and maximum likelihood reconstruction, further revealed the presence of a putative pseudogene that displays hybrid exons similar to tetrapod AQP5 and -1. Ectopic expression of the cloned transcripts in Xenopus laevis oocytes demonstrated that zebrafish aquaporins and Glps transport water or water, glycerol and urea, respectively, whereas DrAqp11b and -12 were not functional in oocytes. Contrary to humans and some rodents, intrachromosomal duplicates of zebrafish AQP8 were water and urea permeable, while the genomic duplicate only transported water. All aquaporin transcripts were expressed in adult tissues and found to have divergent expression patterns. In some tissues, however, redundant expression of transcripts encoding two duplicated paralogs seems to occur.
Conclusion: The zebrafish genome encodes the largest repertoire of functional vertebrate aquaporins with dual paralogy to human isoforms. Our data reveal an early and specific diversification of these integral membrane proteins at the root of the crown-clade of Teleostei. Despite the increase in gene copy number, zebrafish aquaporins mostly retain the substrate specificity characteristic of the tetrapod counterparts. Based upon the integration of phylogenetic, genomic and functional data we propose a new classification for the piscine aquaporin superfamily.
Figures


Similar articles
-
Design and characterization of genetically engineered zebrafish aquaporin-3 mutants highly permeable to the cryoprotectant ethylene glycol.BMC Biotechnol. 2011 Apr 8;11:34. doi: 10.1186/1472-6750-11-34. BMC Biotechnol. 2011. PMID: 21477270 Free PMC article.
-
Phylogenomic and functional analyses of salmon lice aquaporins uncover the molecular diversity of the superfamily in Arthropoda.BMC Genomics. 2015 Aug 19;16(1):618. doi: 10.1186/s12864-015-1814-8. BMC Genomics. 2015. PMID: 26282991 Free PMC article.
-
Piscine aquaporins: an overview of recent advances.J Exp Zool A Ecol Genet Physiol. 2010 Dec 1;313(10):623-50. doi: 10.1002/jez.634. Epub 2010 Aug 17. J Exp Zool A Ecol Genet Physiol. 2010. PMID: 20717996 Review.
-
Aquaporin-11: a channel protein lacking apparent transport function expressed in brain.BMC Biochem. 2006 May 1;7:14. doi: 10.1186/1471-2091-7-14. BMC Biochem. 2006. PMID: 16650285 Free PMC article.
-
Ammonia and urea permeability of mammalian aquaporins.Handb Exp Pharmacol. 2009;(190):327-58. doi: 10.1007/978-3-540-79885-9_17. Handb Exp Pharmacol. 2009. PMID: 19096786 Review.
Cited by
-
Unravelling the Complex Duplication History of Deuterostome Glycerol Transporters.Cells. 2020 Jul 10;9(7):1663. doi: 10.3390/cells9071663. Cells. 2020. PMID: 32664262 Free PMC article.
-
Draft genome assembly of Tenualosa ilisha, Hilsa shad, provides resource for osmoregulation studies.Sci Rep. 2019 Nov 11;9(1):16511. doi: 10.1038/s41598-019-52603-w. Sci Rep. 2019. PMID: 31712633 Free PMC article.
-
Genome-Wide Identification, Characterization and Phylogenetic Analysis of ATP-Binding Cassette (ABC) Transporter Genes in Common Carp (Cyprinus carpio).PLoS One. 2016 Apr 8;11(4):e0153246. doi: 10.1371/journal.pone.0153246. eCollection 2016. PLoS One. 2016. PMID: 27058731 Free PMC article.
-
Aquaporin 8ab is required in zebrafish embryonic intestine development.Acta Biochim Biophys Sin (Shanghai). 2022 Jan 25;54(7):952-960. doi: 10.3724/abbs.2022077. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35880566 Free PMC article.
-
Prediction of aquaporin function by integrating evolutionary and functional analyses.J Membr Biol. 2014 Feb;247(2):107-25. doi: 10.1007/s00232-013-9618-8. Epub 2013 Nov 29. J Membr Biol. 2014. PMID: 24292667 Review.
References
-
- Maurel C, Javot H, Lauvergeat V, Gerbeau P, Tournaire C, Santoni V, Heyes J. Molecular physiology of aquaporins in plants. Int Rev Cytol. 2002;215:105–48. full_text. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

