Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer
- PMID: 20149256
- PMCID: PMC2830182
- DOI: 10.1186/1471-2407-10-40
Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer
Abstract
Background: GPR110 is an orphan G protein-coupled receptor--a receptor without a known ligand, a known signaling pathway, or a known function. Despite the lack of information, one can assume that orphan receptors have important biological roles. In a retroviral insertion mutagenesis screen in the mouse, we identified GPR110 as an oncogene. This prompted us to study the potential isoforms that can be gleaned from known GPR110 transcripts, and the expression of these isoforms in normal and transformed human tissues.
Methods: Various epitope-tagged isoforms of GPR110 were expressed in cell lines and assayed by western blotting to determine cleavage, surface localization, and secretion patterns. GPR110 transcript and protein levels were measured in lung and prostate cancer cell lines and clinical samples, respectively, by quantitative PCR and immunohistochemistry.
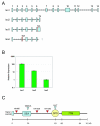
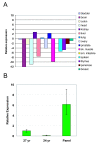
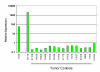
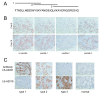
Results: We found four potential splice variants of GPR110. Of these variants, we confirmed three as being expressed as proteins on the cell surface. Isoform 1 is the canonical form, with a molecular mass of about 100 kD. Isoforms 2 and 3 are truncated products of isoform 1, and are 25 and 23 kD, respectively. These truncated isoforms lack the seven-span transmembrane domain characteristic of GPR proteins and thus are not likely to be membrane anchored; indeed, isoform 2 can be secreted. Compared with the median gene expression of approximately 200 selected genes, GPR110 expression was low in most tissues. However, it had higher than average gene expression in normal kidney tissue and in prostate tissues originating from older donors. Although identified as an oncogene in murine T lymphomas, GPR110 is greatly overexpressed in human lung and prostate cancers. As detected by immunohistochemistry, GPR110 was overexpressed in 20 of 27 (74%) lung adenocarcinoma tissue cores and in 17 of 29 (59%) prostate adenocarcinoma tissue cores. Additionally, staining with a GPR110 antibody enabled us to differentiate between benign prostate hyperplasia and potential incipient malignancy.
Conclusion: Our work suggests a role for GPR110 in tumor physiology and supports it as a potential therapeutic candidate and disease marker for both lung and prostate cancer.
Figures






References
-
- Filmore D. It's a GPCR world. Modern Drug Discovery. 2004;7(11):24–28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

