Poly(lactide-co-glycolide) nanoparticle assembly for highly efficient delivery of potent therapeutic agents from medical devices
- PMID: 20149428
- PMCID: PMC2837119
- DOI: 10.1016/j.biomaterials.2010.01.048
Poly(lactide-co-glycolide) nanoparticle assembly for highly efficient delivery of potent therapeutic agents from medical devices
Abstract
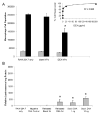
Controlled delivery of therapeutic agents from medical devices can improve their safety and effectiveness in vivo, by ameliorating the surrounding tissue responses and thus maintaining the functional integrity of the devices. Previously, we presented a new method for providing simultaneous controlled delivery from medical devices, by surface assembly of biodegradable polymer nanoparticles (NPs) encapsulating fluorescent dyes. Here, we continue our investigation with NPs loaded with therapeutic agents, dexamethasone (DEX) or plasmid DNA, and evaluated the bioactivity of the released molecules with macrophage cells associated with inflammation. Over a period of one week, NPs encapsulating DEX released 24.9+/-0.8ng from the probe surface and was successful at suppressing macrophage cell growth by 40+/-10%. This percentage of suppression corresponded to approximately 100% drug delivery efficiency, in comparison with the unencapsulated drug. DNA NP coatings, in contrast, released approximately 1ng of plasmid DNA and were effective at transfecting macrophage cells to express the luciferase gene at 300+/-200 relative luminescence/mg total protein. This amount of luciferase activity corresponded to 100% gene delivery efficiency. Thus, NP coatings were capable of providing continuous release of bioactive agents in sufficient quantities to induce relevant biological effects in cell culture studies. These coatings also remained intact, even after 14 days of incubation with phosphate buffered saline. Although the maximum loading for NP coatings is inherently lower than the more established matrix coating, our study suggests that the NP coatings are a more versatile and efficient approach toward drug delivery or gene delivery from a medical device surface and are perhaps best suited for continuous release of highly potent therapeutic agents.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Simultaneous release of multiple molecules from poly(lactide-co-glycolide) nanoparticles assembled onto medical devices.Biomaterials. 2009 Oct;30(28):4889-97. doi: 10.1016/j.biomaterials.2009.05.074. Epub 2009 Jul 9. Biomaterials. 2009. PMID: 19592089 Free PMC article.
-
Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile.Int J Nanomedicine. 2017 Jan 27;12:935-947. doi: 10.2147/IJN.S121881. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28184163 Free PMC article.
-
On the design of in situ forming biodegradable parenteral depot systems based on insulin loaded dialkylaminoalkyl-amine-poly(vinyl alcohol)-g-poly(lactide-co-glycolide) nanoparticles.J Control Release. 2007 Nov 6;123(2):131-40. doi: 10.1016/j.jconrel.2007.08.004. Epub 2007 Aug 16. J Control Release. 2007. PMID: 17854938
-
Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery.J Pharm Sci. 2008 Jul;97(7):2448-61. doi: 10.1002/jps.21215. J Pharm Sci. 2008. PMID: 17918737 Review.
-
Drug delivery into the brain using poly(lactide-co-glycolide) microspheres.Expert Opin Drug Deliv. 2005 Mar;2(2):363-76. doi: 10.1517/17425247.2.2.363. Expert Opin Drug Deliv. 2005. PMID: 16296760 Review.
Cited by
-
Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent.Tissue Eng Part A. 2018 Jul;24(13-14):1157-1166. doi: 10.1089/ten.TEA.2017.0404. Epub 2018 Mar 13. Tissue Eng Part A. 2018. PMID: 29431053 Free PMC article.
-
Synthetic Biomaterials from Metabolically Derived Synthons.Chem Rev. 2016 Feb 24;116(4):2664-704. doi: 10.1021/acs.chemrev.5b00465. Epub 2016 Jan 29. Chem Rev. 2016. PMID: 26821863 Free PMC article. Review.
-
Nanoparticles and their potential for application in bone.Int J Nanomedicine. 2012;7:4545-57. doi: 10.2147/IJN.S34127. Epub 2012 Aug 17. Int J Nanomedicine. 2012. PMID: 22923992 Free PMC article. Review.
-
Steroid-Loaded Hemostatic Nanoparticles Combat Lung Injury after Blast Trauma.ACS Macro Lett. 2015 Apr 21;4(4):387-391. doi: 10.1021/acsmacrolett.5b00061. Epub 2015 Mar 23. ACS Macro Lett. 2015. PMID: 27668129 Free PMC article.
-
Characterization of immunogenicity of avian influenza antigens encapsulated in PLGA nanoparticles following mucosal and subcutaneous delivery in chickens.PLoS One. 2018 Nov 1;13(11):e0206324. doi: 10.1371/journal.pone.0206324. eCollection 2018. PLoS One. 2018. PMID: 30383798 Free PMC article.
References
-
- Cui XY, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24(5):777–87. - PubMed
-
- Zhong Y, Yu X, Gilbert R, Bellamkonda RV. Stabilizing electrode-host interfaces: a tissue engineering approach. J Rehabil Res Dev. 2001;38(6):627–32. - PubMed
-
- Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28(25):3594–607. - PubMed
-
- Cui XY, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, et al. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56(2):261–72. - PubMed
-
- Kam L, Shain W, Turner JN, Bizios R. Selective adhesion of astrocytes to surfaces modified with immobilized peptides. Biomaterials. 2002;23(2):511–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

