Synthesis, biophysical properties and pharmacokinetics of ultrahigh molecular weight tense and relaxed state polymerized bovine hemoglobins
- PMID: 20149433
- PMCID: PMC2837140
- DOI: 10.1016/j.biomaterials.2010.01.072
Synthesis, biophysical properties and pharmacokinetics of ultrahigh molecular weight tense and relaxed state polymerized bovine hemoglobins
Abstract
Hemoglobin-based oxygen carriers (HBOC) are currently being developed as red blood cell (RBC) substitutes for use in transfusion medicine. Despite significant commercial development, late stage clinical results of polymerized hemoglobin (PolyHb) solutions hamper development. We synthesized two types of PolyHbs with ultrahigh molecular weights: tense (T) state PolyHb (M(W)=16.59 MDa and P(50)=41 mmHg) and relaxed (R) state PolyHb (M(W)=26.33 MDa and P(50)=0.66 mmHg). By maintaining Hb in either the T- or R-state during the polymerization reaction, we were able to synthesize ultrahigh molecular weight PolyHbs in distinct quaternary states with no tetrameric Hb, high viscosity, low colloid osmotic pressure and the ability to maintain O(2) dissociation, CO association and NO dioxygenation reactions. The PolyHbs elicited some in vitro RBC aggregation that was less than 6% dextran (500 kDa) but more than 5% human serum albumin. In vitro, T-state PolybHb autoxidized faster than R-state PolybHb as expected from previously reported studies, conversely, when administered to guinea pigs as a 20% exchange transfusion, R-state PolybHb oxidized faster and to a greater extent than T-state PolybHb, suggesting a more complex oxidative processes in vivo. Our findings also demonstrate that T-state PolybHb exhibited a longer circulating half-life, slower clearance and longer systemic exposure time compared to R-state PolybHb.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
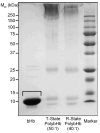
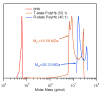
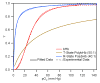
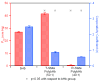
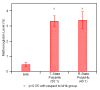



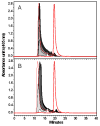
 ) bHb, (
) bHb, ( ) T-state PolybHb and plasma samples from the end of transfusion to 48 hours (shown as dark grey fading to white). (B) Size exclusion chromatography of (
) T-state PolybHb and plasma samples from the end of transfusion to 48 hours (shown as dark grey fading to white). (B) Size exclusion chromatography of ( ) bHb, (
) bHb, ( ) R-PolybBv and plasma samples from the end of transfusion to 24 hours (shown as dark grey fading to white).
) R-PolybBv and plasma samples from the end of transfusion to 24 hours (shown as dark grey fading to white).
References
-
- Greenburg AG. Benefits and risks of blood transfusion in surgical patients. World J Surg. 1996;20(9):1189–93. - PubMed
-
- Finucane ML, Slovic P, Mertz CK. Public perception of the risk of blood transfusion. Transfusion. 2000;40(8):1017–22. - PubMed
-
- Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289(8):959–62. - PubMed
-
- Stramer SL. Current risks of transfusion-transmitted agents - A review. Arch Pathol Lab Med. 2007;131(5):702–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

