Biohybrid thin films for measuring contractility in engineered cardiovascular muscle
- PMID: 20149449
- PMCID: PMC2838170
- DOI: 10.1016/j.biomaterials.2010.01.079
Biohybrid thin films for measuring contractility in engineered cardiovascular muscle
Abstract
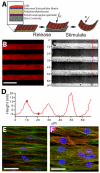
In vitro cardiovascular disease models need to recapitulate tissue-scale function in order to provide in vivo relevance. We have developed a new method for measuring the contractility of engineered cardiovascular smooth and striated muscle in vitro during electrical and pharmacological stimulation. We present a growth theory-based finite elasticity analysis for calculating the contractile stresses of a 2D anisotropic muscle tissue cultured on a flexible synthetic polymer thin film. Cardiac muscle engineered with neonatal rat ventricular myocytes and paced at 0.5 Hz generated stresses of 9.2 +/- 3.5 kPa at peak systole, similar to measurements of the contractility of papillary muscle from adult rats. Vascular tissue engineered with human umbilical arterial smooth muscle cells maintained a basal contractile tone of 13.1 +/- 2.1 kPa and generated another 5.1 +/- 0.8 kPa when stimulated with endothelin-1. These data suggest that this method may be useful in assessing the efficacy and safety of pharmacological agents on cardiovascular tissue.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures





References
-
- Rohr S, Scholly DM, Kleber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991;68:114–30. - PubMed
-
- Gillis AM, Fast VG, Rohr S, Kleber AG. Spatial changes in transmembrane potential during extracellular electrical shocks in cultured monolayers of neonatal rat ventricular myocytes. Circ Res. 1996;79:676–90. - PubMed
-
- Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy - A model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:E45–E54. - PubMed
-
- Entcheva E, Bien H, Yin LH, Chung CY, Farrell M, Kostov Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials. 2004;25:5753–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

