Virus particle maturation: insights into elegantly programmed nanomachines
- PMID: 20149636
- PMCID: PMC2854226
- DOI: 10.1016/j.sbi.2010.01.004
Virus particle maturation: insights into elegantly programmed nanomachines
Abstract
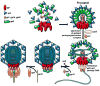
Similar modes of virus maturation have been observed in dsDNA bacteriophages and the structurally related herpes viruses and some type of maturation occur in most animal viruses. Recently a variety of biophysical studies of maturation intermediates of bacteriophages P22, lambda, and HK97 have suggested an energy landscape that drives the transitions and structure-based mechanisms for its formation. Near-atomic resolution models of subunit tertiary structures in an early intermediate of bacteriophage HK97 maturation revealed a remarkable distortion of the secondary structures when compared to the mature particle. Scaffolding proteins may induce the distortion that is maintained by quaternary structure interactions following scaffold release, making the intermediate particle metastable.
Figures


References
-
- Katen S, Zlotnick A. The thermodynamics of virus capsid assembly. In: Johnson ML, Holt JM, Ackers GK, editors. Methods in Enzymology: Biothermodynamics, part A. Vol. 395. Academic Press; 2009. pp. 417–455. An excellent review of the thermodynamics and kinetics of assembly of icosahedral viruses that explicitly addresses the role of weak subunit interactions in successful particle assembly. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

