Activation of the A(3) adenosine receptor inhibits fMLP-induced Rac activation in mouse bone marrow neutrophils
- PMID: 20149782
- PMCID: PMC2847012
- DOI: 10.1016/j.bcp.2010.02.002
Activation of the A(3) adenosine receptor inhibits fMLP-induced Rac activation in mouse bone marrow neutrophils
Abstract
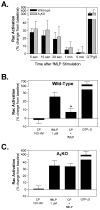
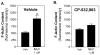
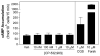
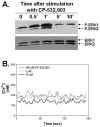
Adenosine is released from injured or hypoxic tissues where it exerts numerous anti-inflammatory effects including suppression of neutrophil functions. Although most previous work has implicated the A(2A)AR, we have recently shown that selective activation of the abundantly expressed A(3)AR inhibits neutrophil superoxide production and chemotaxis providing a potential mechanistic explanation for the efficacy of A(3)AR agonists in experimental animal models of inflammation. In this study, we hypothesized that the A(3)AR suppresses neutrophil functions by inhibiting the monomeric GTPase Rac, a central regulator of chemokine-directed neutrophil migration and superoxide production. We found that pre-treating neutrophils with the highly selective A(3)AR agonist CP-532,903 reduced fMLP-induced Rac activation using an ELISA-based assay that detects all three Rac isoforms. CP-532,903 also inhibited fMLP-induced F-actin formation, a downstream effector function of Rac relevant to neutrophil migration, but not activation of ERK1/2 or p38. Pre-treating neutrophils with CP-532,903 did not stimulate cAMP production or alter fMLP-induced calcium transients, implicating that A(3)AR stimulation does not inhibit Rac activation or neutrophil activities by suppressing Ca(2+) signaling, elevating the intracellular concentration of cAMP, or by cross-desensitizing fMLP receptors. Our results suggest that activation of the A(3)AR signals to suppress neutrophil functions by interfering with the monomeric GTPase Rac, thus contributing to the ant-inflammatory actions of adenosine.
2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils.J Pharmacol Exp Ther. 2011 Sep;338(3):1004-12. doi: 10.1124/jpet.111.181792. Epub 2011 Jun 21. J Pharmacol Exp Ther. 2011. PMID: 21693629 Free PMC article.
-
Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils.Mol Pharmacol. 2008 Sep;74(3):685-96. doi: 10.1124/mol.108.048066. Epub 2008 Jun 26. Mol Pharmacol. 2008. PMID: 18583455 Free PMC article.
-
Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions.J Immunol. 2002 Nov 1;169(9):5043-51. doi: 10.4049/jimmunol.169.9.5043. J Immunol. 2002. PMID: 12391220
-
Disturbed granulocyte macrophage-colony stimulating factor priming of phosphatidylinositol 3,4,5-trisphosphate accumulation and Rac activation in fMLP-stimulated neutrophils from patients with myelodysplasia.J Leukoc Biol. 2004 Jul;76(1):254-62. doi: 10.1189/jlb.0204071. Epub 2004 Apr 23. J Leukoc Biol. 2004. PMID: 15107457
-
The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions.J Immunol. 2003 Oct 15;171(8):4425-30. doi: 10.4049/jimmunol.171.8.4425. J Immunol. 2003. PMID: 14530369
Cited by
-
Characterization of Dual-Acting A3 Adenosine Receptor Positive Allosteric Modulators That Preferentially Enhance Adenosine-Induced Gαi3 and GαoA Isoprotein Activation.ACS Pharmacol Transl Sci. 2022 Jul 15;5(8):625-641. doi: 10.1021/acsptsci.2c00076. eCollection 2022 Aug 12. ACS Pharmacol Transl Sci. 2022. PMID: 35983277 Free PMC article.
-
Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis.Circulation. 2011 Nov 22;124(21):2338-49. doi: 10.1161/CIRCULATIONAHA.111.041418. Epub 2011 Oct 17. Circulation. 2011. PMID: 22007077 Free PMC article.
-
Adenosine 2B Receptor Signaling Impairs Vaccine-Mediated Protection Against Pneumococcal Infection in Young Hosts by Blunting Neutrophil Killing of Antibody-Opsonized Bacteria.Vaccines (Basel). 2025 Apr 15;13(4):414. doi: 10.3390/vaccines13040414. Vaccines (Basel). 2025. PMID: 40333334 Free PMC article.
-
A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils.J Pharmacol Exp Ther. 2011 Sep;338(3):1004-12. doi: 10.1124/jpet.111.181792. Epub 2011 Jun 21. J Pharmacol Exp Ther. 2011. PMID: 21693629 Free PMC article.
-
New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors.Purinergic Signal. 2012 Sep;8(3):587-98. doi: 10.1007/s11302-012-9311-x. Epub 2012 Apr 15. Purinergic Signal. 2012. PMID: 22528684 Free PMC article. Review.
References
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. - PubMed
-
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

