Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women
- PMID: 20149794
- PMCID: PMC2854893
- DOI: 10.1016/j.jim.2010.02.002
Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women
Abstract
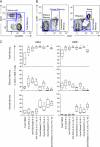
Cervical cytobrushing is a useful and non-invasive method for obtaining mucosal mononuclear cells from the female genital tract, but yields few cells. The aim of this study was to compare in vitro expansion protocols (anti-CD3, anti-CD3/CD28 or Dynal anti-CD3/CD28 beads) and cytokine combinations (IL-2, IL-7 and IL-15) to improve cervical T cell yields and viability. Eighteen HIV-infected women were included in this study to compare methods for polyclonal expansion of T cells from the female genital tract and blood. Comparison of T cell yields, viability and maturational status (by differential staining with CD45RO, CCR7 and CD27) was determined following 7 days of in vitro expansion. Anti-CD3 and IL-2 resulted in a 4.5-fold (range 3.7-5.3) expansion of cervical CD3+ T cells in 7 days compared to day 0. Inclusion of anti-CD28 or addition of IL-7 and IL-15 to this combination did not improve expansion. Culturing cells with Dynal beads (1:1) and IL-2, IL-7 and IL-15 gave rise to the highest yields after 7 days in both blood (7.1-fold) and cervix (5.6-fold). While expansion with anti-CD3 led to the accumulation of effector memory T cells (CD45RO+CCR7-CD27-), expansion with Dynabeads selected for accumulation of central memory T cells (CD45RO+CCR7+CD27+). We conclude that in vitro expansion with Dynabeads (1:1) in the presence of IL-2, IL-7 and IL-15 resulted in the greatest increase in viable T cells from both blood and cytobrush. Irrespective of the expansion method used, the T cell memory profile was altered following expansion.
2010 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract.Immunology. 2009 Sep;128(1 Suppl):e746-57. doi: 10.1111/j.1365-2567.2009.03077.x. Epub 2009 Mar 26. Immunology. 2009. PMID: 19740336 Free PMC article.
-
Stability and transport of cervical cytobrushes for isolation of mononuclear cells from the female genital tract.J Immunol Methods. 2011 Mar 31;367(1-2):47-55. doi: 10.1016/j.jim.2011.01.013. Epub 2011 Feb 12. J Immunol Methods. 2011. PMID: 21324321 Free PMC article.
-
Polyclonal expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract.Immunology. 2010 May;130(1):23-33. doi: 10.1111/j.1365-2567.2009.03172.x. Epub 2009 Aug 17. Immunology. 2010. PMID: 20201983 Free PMC article.
-
Comprehensive Approach for Identifying the T Cell Subset Origin of CD3 and CD28 Antibody-Activated Chimeric Antigen Receptor-Modified T Cells.J Immunol. 2017 Jul 1;199(1):348-362. doi: 10.4049/jimmunol.1601494. Epub 2017 May 26. J Immunol. 2017. PMID: 28550199 Free PMC article.
-
Regional and mucosal memory T cells.Nat Immunol. 2011 Jun;12(6):485-91. doi: 10.1038/ni.2029. Nat Immunol. 2011. PMID: 21739671 Free PMC article. Review.
Cited by
-
Evaluation of a quantitative real-time PCR assay to measure HIV-specific mucosal CD8+ T cell responses in the cervix.PLoS One. 2010 Oct 7;5(10):e13077. doi: 10.1371/journal.pone.0013077. PLoS One. 2010. PMID: 20949096 Free PMC article.
-
Immune responses to HIV in the female reproductive tract, immunologic parallels with the gastrointestinal tract, and research implications.Am J Reprod Immunol. 2011 Mar;65(3):230-41. doi: 10.1111/j.1600-0897.2010.00948.x. Epub 2011 Jan 12. Am J Reprod Immunol. 2011. PMID: 21223420 Free PMC article. Review.
-
Impact of HIV-ART on the restoration of Th17 and Treg cells in blood and female genital mucosa.Sci Rep. 2019 Feb 13;9(1):1978. doi: 10.1038/s41598-019-38547-1. Sci Rep. 2019. PMID: 30760809 Free PMC article.
-
Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract.PLoS One. 2014 Jun 4;9(6):e98593. doi: 10.1371/journal.pone.0098593. eCollection 2014. PLoS One. 2014. PMID: 24896815 Free PMC article.
-
Vaccine applications of flow cytometry.Methods. 2012 Jul;57(3):383-91. doi: 10.1016/j.ymeth.2012.01.001. Epub 2012 Jan 10. Methods. 2012. PMID: 22251671 Free PMC article. Review.
References
-
- Boise L.H., Minn A.J., Noel P.J., June C.H., Accavitti M.A., Lindsten T., Thompson C.B. CD28 costimulation can promote T cell survival by enhancing the expression of bcl-XL. Immunity. 1995;3:87. - PubMed
-
- Burgers W.A., Riou C., Mlotshwa M., Maenetje P., de Assis Rosa D., Brenchley J., Mlisana K., Douek D.C., Koup R., Roederer M., de Bruyn G., Karim S.A., Williamson C., Gray C.M., the CAPRISA 002 Acute Infection Study Team Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J. Immunol. 2009;182:4751. - PMC - PubMed
-
- Coombs R.W., Wright D.J., Reichelderfer P.S., Burns D.N., Cohn J., Cu-Uvin S., Baron P.A., Cohen M.H., Landay A.L., Lewis S., Kovacs A., Women's Health Study 001 Team Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J. Infect. Dis. 2001;184:1187. - PubMed
-
- Geginat J., Sallusto F., Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory and effector memory CD4+ T cells. Pathol. Biol. 2003;51:64. (Paris) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

