Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory
- PMID: 20149820
- PMCID: PMC2894302
- DOI: 10.1016/j.neubiorev.2010.02.001
Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory
Abstract
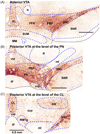
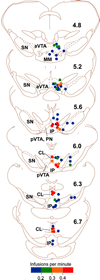
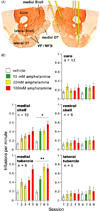
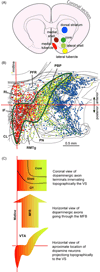
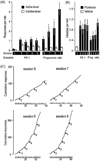
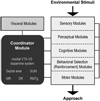
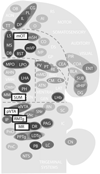
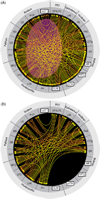
Reductionist attempts to dissect complex mechanisms into simpler elements are necessary, but not sufficient for understanding how biological properties like reward emerge out of neuronal activity. Recent studies on intracranial self-administration of neurochemicals (drugs) found that rats learn to self-administer various drugs into the mesolimbic dopamine structures-the posterior ventral tegmental area, medial shell nucleus accumbens and medial olfactory tubercle. In addition, studies found roles of non-dopaminergic mechanisms of the supramammillary, rostromedial tegmental and midbrain raphe nuclei in reward. To explain intracranial self-administration and related effects of various drug manipulations, I outlined a neurobiological theory claiming that there is an intrinsic central process that coordinates various selective functions (including perceptual, visceral, and reinforcement processes) into a global function of approach. Further, this coordinating process for approach arises from interactions between brain structures including those structures mentioned above and their closely linked regions: the medial prefrontal cortex, septal area, ventral pallidum, bed nucleus of stria terminalis, preoptic area, lateral hypothalamic areas, lateral habenula, periaqueductal gray, laterodorsal tegmental nucleus and parabrachical area.
Published by Elsevier Ltd.
Figures



References
-
- Albert R, Jeong H, Barabasi A-L. Error and attack tolerance of complex networks. Nature. 2000;406:378–382. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. - PubMed
-
- Allen CN, Crawford IL. GABAergic agents in the medial septal nucleus affect hippocampal theta rhythm and acetylcholine utilization. Brain Res. 1984;322:261–267. - PubMed
-
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

