Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium
- PMID: 20150244
- PMCID: PMC2867431
- DOI: 10.1152/ajpgi.00293.2009
Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium
Abstract
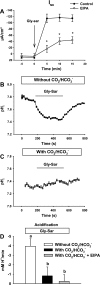
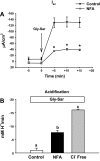
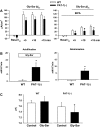
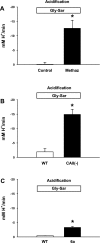
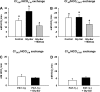
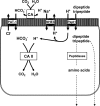
The majority of dietary amino acids are absorbed via the H(+)-di-/tripeptide transporter Pept1 of the small intestine. Proton influx via Pept1 requires maintenance of intracellular pH (pH(i)) to sustain the driving force for peptide absorption. The apical membrane Na(+)/H(+) exchanger Nhe3 plays a major role in minimizing epithelial acidification during H(+)-di-/tripeptide absorption. However, the contributions of HCO(3)(-)-dependent transporters to this process have not been elucidated. In this study, we investigate the role of putative anion transporter-1 (Pat-1), an apical membrane anion exchanger, in epithelial pH(i) regulation during H(+)-peptide absorption. Using wild-type (WT) and Pat-1(-) mice, Ussing chambers were employed to measure the short-circuit current (I(sc)) associated with Pept1-mediated glycyl-sarcosine (Gly-Sar) absorption. Microfluorometry was used to measure pH(i) and Cl(-)/HCO(3)(-) exchange in the upper villous epithelium. In CO(2)/HCO(3)(-)-buffered Ringers, WT small intestine showed significant Gly-Sar-induced I(sc) and efficient pH(i) regulation during pharmacological inhibition of Nhe3 activity. In contrast, epithelial acidification and reduced I(sc) response to Gly-Sar exposure occurred during pharmacological inhibition of Cl(-)/HCO(3)(-) exchange and in the Pat-1(-) intestine. Pat-1 interacts with carbonic anhydrase II (CAII), and studies using CAII(-) intestine or the pharmacological inhibitor methazolamide on WT intestine resulted in increased epithelial acidification during Gly-Sar exposure. Increased epithelial acidification during Gly-Sar exposure also occurred in WT intestine during inhibition of luminal extracellular CA activity. Measurement of Cl(-)/HCO(3)(-) exchange in the presence of Gly-Sar revealed an increased rate of Cl(-)(OUT)/HCO(3)(-)(IN) exchange that was both Pat-1 dependent and CA dependent. In conclusion, Pat-1 Cl(-)/HCO(3)(-) exchange contributes to pH(i) regulation in the villous epithelium during H(+)-dipeptide absorption, possibly by providing a HCO(3)(-) import pathway.
Figures
Similar articles
-
PAT-1 (Slc26a6) is the predominant apical membrane Cl-/HCO3- exchanger in the upper villous epithelium of the murine duodenum.Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1079-88. doi: 10.1152/ajpgi.00354.2006. Epub 2006 Dec 14. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17170027
-
Functional activity of Pat-1 (Slc26a6) Cl(−)/HCO₃(−) exchange in the lower villus epithelium of murine duodenum.Acta Physiol (Oxf). 2011 Jan;201(1):21-31. doi: 10.1111/j.1748-1716.2010.02210.x. Epub 2010 Nov 12. Acta Physiol (Oxf). 2011. PMID: 20969732 Free PMC article.
-
Role of down-regulated in adenoma anion exchanger in HCO3- secretion across murine duodenum.Gastroenterology. 2009 Mar;136(3):893-901. doi: 10.1053/j.gastro.2008.11.016. Epub 2008 Nov 8. Gastroenterology. 2009. PMID: 19121635 Free PMC article.
-
Effects of Slc26a6 deletion and CFTR inhibition on HCO3- secretion by mouse pancreatic duct.J Med Invest. 2009;56 Suppl:332-5. doi: 10.2152/jmi.56.332. J Med Invest. 2009. PMID: 20224218 Review.
-
High rates of HCO3- secretion and Cl- absorption against adverse gradients in the marine teleost intestine: the involvement of an electrogenic anion exchanger and H+-pump metabolon?J Exp Biol. 2009 Jun;212(Pt 11):1684-96. doi: 10.1242/jeb.027730. J Exp Biol. 2009. PMID: 19448078 Review.
Cited by
-
The anion exchanger PAT-1 (Slc26a6) does not participate in oxalate or chloride transport by mouse large intestine.Pflugers Arch. 2021 Jan;473(1):95-106. doi: 10.1007/s00424-020-02495-x. Epub 2020 Nov 17. Pflugers Arch. 2021. PMID: 33205229 Free PMC article.
-
Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets.Am J Respir Cell Mol Biol. 2013 Nov;49(5):837-44. doi: 10.1165/rcmb.2012-0433OC. Am J Respir Cell Mol Biol. 2013. PMID: 23782101 Free PMC article.
-
Cell-specific effects of luminal acid, bicarbonate, cAMP, and carbachol on transporter trafficking in the intestine.Am J Physiol Gastrointest Liver Physiol. 2012 Oct 15;303(8):G937-50. doi: 10.1152/ajpgi.00452.2011. Epub 2012 Aug 30. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22936272 Free PMC article.
-
Bicarbonate secretion and acid/base sensing by the intestine.Pflugers Arch. 2024 Apr;476(4):593-610. doi: 10.1007/s00424-024-02914-3. Epub 2024 Feb 19. Pflugers Arch. 2024. PMID: 38374228 Free PMC article. Review.
-
BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity.PLoS One. 2014 Nov 3;9(11):e109972. doi: 10.1371/journal.pone.0109972. eCollection 2014. PLoS One. 2014. PMID: 25365555 Free PMC article.
References
-
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988 - PubMed
-
- Buyse M, Tsocas A, Walker F, Merlin D, Bado A. PEPT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol 283: C1795–C1800, 2002 - PubMed
-
- Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol Gastrointest Liver Physiol 270: G259–G267, 1996 - PubMed
-
- Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

