Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves
- PMID: 20150245
- PMCID: PMC2867417
- DOI: 10.1152/ajpgi.00260.2009
Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves
Abstract
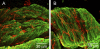
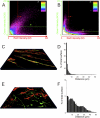
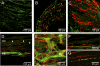
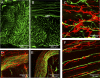
The morphology of interstitial cells of Cajal (ICC) in the circular muscle layer of the cynomolgus monkey internal anal sphincter (IAS) and rectum and their relationship to sympathetic and nitrergic nerves were compared by dual-labeling immunohistochemistry. Contractile studies confirmed that nitrergic nerves participate in neural inhibition in both regions whereas sympathetic nerves serve as excitatory motor nerves only in the IAS. Muscle bundles extended from myenteric to submucosal edge in rectum but in the IAS bundles were further divided into "minibundles" each surrounded by connective tissue. Dual labeling of KIT and smooth muscle myosin revealed KIT-positive stellate-shaped ICC (ICC-IAS) within each minibundle. In the rectum intramuscular ICC (ICC-IM) were spindle shaped whereas stellate-shaped ICC were located at the myenteric surface (ICC-MY). ICC were absent from both the myenteric and submucosal surfaces of the IAS. Nitrergic nerves (identified with anti-neuronal nitric oxide synthase antibodies or NADPH diaphorase activity) and sympathetic nerves (identified with anti-tyrosine hydroxylase antibody) each formed a plexus at the myenteric surface of the rectum but not the IAS. Intramuscular neuronal nitric oxide synthase- and tyrosine hydroxylase-positive fibers were present in both regions but were only closely associated with ICC-IM in rectum. Minimal association was also noted between ICC-IAS and cells expressing the nonspecific neuronal marker PGP9.5. In conclusion, the morphology of rectal ICC-IM and ICC-MY is similar to that described elsewhere in the gastrointestinal tract whereas ICC-IAS are unique. The distribution of stellate-shaped ICC-IAS throughout the musculature and their absence from both the myenteric and submucosal surfaces suggest that ICC-IAS may serve as pacemaker cells in this muscle whereas their limited relationship to nerves suggests that they are not involved in neuromuscular transmission. Additionally, the presence of numerous minibundles, each containing both ICC-IAS and nerves, suggests that this muscle functions as a multiunit type muscle.
Figures





Similar articles
-
Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter.Cell Tissue Res. 2011 Apr;344(1):17-30. doi: 10.1007/s00441-011-1138-1. Epub 2011 Feb 22. Cell Tissue Res. 2011. PMID: 21337122 Free PMC article.
-
Comparison of inhibitory neuromuscular transmission in the Cynomolgus monkey IAS and rectum: special emphasis on differences in purinergic transmission.J Physiol. 2018 Nov;596(22):5319-5341. doi: 10.1113/JP275437. Epub 2018 Oct 13. J Physiol. 2018. PMID: 30198065 Free PMC article.
-
Changes in neuromuscular transmission in the W/W(v) mouse internal anal sphincter.Neurogastroenterol Motil. 2012 Jan;24(1):e41-55. doi: 10.1111/j.1365-2982.2011.01806.x. Epub 2011 Nov 10. Neurogastroenterol Motil. 2012. PMID: 22074497 Free PMC article.
-
The functional role of intramuscular interstitial cells of Cajal in the stomach.J Smooth Muscle Res. 2011;47(2):47-53. doi: 10.1540/jsmr.47.47. J Smooth Muscle Res. 2011. PMID: 21757854 Review.
-
The interstitial cells of Cajal and a gastroenteric pacemaker system.Arch Histol Cytol. 2002 Mar;65(1):1-26. doi: 10.1679/aohc.65.1. Arch Histol Cytol. 2002. PMID: 12002607 Review.
Cited by
-
Control of Motility in the Internal Anal Sphincter.J Neurogastroenterol Motil. 2019 Apr 30;25(2):189-204. doi: 10.5056/jnm18172. J Neurogastroenterol Motil. 2019. PMID: 30827084 Free PMC article.
-
Ca2+ signalling behaviours of intramuscular interstitial cells of Cajal in the murine colon.J Physiol. 2019 Jul;597(14):3587-3617. doi: 10.1113/JP278036. Epub 2019 Jun 13. J Physiol. 2019. PMID: 31124144 Free PMC article.
-
The Prevalence of Enteropathy Symptoms from the Lower Gastrointestinal Tract and the Evaluation of Anorectal Function in Diabetes Mellitus Patients.J Clin Med. 2021 Jan 22;10(3):415. doi: 10.3390/jcm10030415. J Clin Med. 2021. PMID: 33499216 Free PMC article.
-
Interstitial cells in the primate gastrointestinal tract.Cell Tissue Res. 2012 Nov;350(2):199-213. doi: 10.1007/s00441-012-1468-7. Epub 2012 Aug 3. Cell Tissue Res. 2012. PMID: 22864981 Free PMC article.
-
Modulation of intracellular calcium activity in interstitial cells of Cajal by inhibitory neural pathways within the internal anal sphincter.Am J Physiol Gastrointest Liver Physiol. 2024 Sep 1;327(3):G382-G404. doi: 10.1152/ajpgi.00309.2023. Epub 2024 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38860285 Free PMC article.
References
-
- Baumgarten HG, Holstein AF, Stelzner F. [Differences in the innervation of the large intestine and the internal sphincter of the anus in mammals and humans]. Verh Anat Ges 66: 43–47, 1971 - PubMed
-
- Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil 18: 507–519, 2006 - PubMed
-
- Brading AF, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res 152: 345–358, 2006 - PubMed
-
- Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol 150: 223–228, 1993 - PubMed
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res 290: 11–20, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

