Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides
- PMID: 20150258
- PMCID: PMC4111897
- DOI: 10.2967/jnumed.109.068007
Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides
Abstract
Targeted contrast-enhanced ultrasound imaging is increasingly being recognized as a powerful imaging tool for the detection and quantification of tumor angiogenesis at the molecular level. The purpose of this study was to develop and test a new class of targeting ligands for targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with small, conformationally constrained peptides that can be coupled to the surface of ultrasound contrast agents.
Methods: Directed evolution was used to engineer a small, disulfide-constrained cystine knot (knottin) peptide that bound to alpha(v)beta(3) integrins with a low nanomolar affinity (Knottin(Integrin)). A targeted contrast-enhanced ultrasound imaging contrast agent was created by attaching Knottin(Integrin) to the shell of perfluorocarbon-filled microbubbles (MB-Knottin(Integrin)). A knottin peptide with a scrambled sequence was used to create control microbubbles (MB-Knottin(Scrambled)). The binding of MB-Knottin(Integrin) and MB-Knottin(Scrambled) to alpha(v)beta(3) integrin-positive cells and control cells was assessed in cell culture binding experiments and compared with that of microbubbles coupled to an anti-alpha(v)beta(3) integrin monoclonal antibody (MB(alphavbeta3)) and microbubbles coupled to the peptidomimetic agent c(RGDfK) (MB(cRGD)). The in vivo imaging signals of contrast-enhanced ultrasound with the different types of microbubbles were quantified in 42 mice bearing human ovarian adenocarcinoma xenograft tumors by use of a high-resolution 40-MHz ultrasound system.
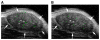
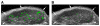
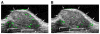
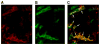
Results: MB-Knottin(Integrin) attached significantly more to alpha(v)beta(3) integrin-positive cells (1.76 +/- 0.49 [mean +/- SD] microbubbles per cell) than to control cells (0.07 +/- 0.006). Control MB-Knottin(Scrambled) adhered less to alpha(v)beta(3) integrin-positive cells (0.15 +/- 0.12) than MB-Knottin(Integrin). After blocking of integrins, the attachment of MB-Knottin(Integrin) to alpha(v)beta(3) integrin-positive cells decreased significantly. The in vivo ultrasound imaging signal was significantly higher after the administration of MB-Knottin(Integrin) than after the administration of MB(alphavbeta3) or control MB-Knottin(Scrambled). After in vivo blocking of integrin receptors, the imaging signal after the administration of MB-Knottin(Integrin) decreased significantly (by 64%). The imaging signals after the administration of MB-Knottin(Integrin) were not significantly different in the groups of tumor-bearing mice imaged with MB-Knottin(Integrin) and with MB(cRGD). Ex vivo immunofluorescence confirmed integrin expression on endothelial cells of human ovarian adenocarcinoma xenograft tumors.
Conclusion: Integrin-binding knottin peptides can be conjugated to the surface of microbubbles and used for in vivo targeted contrast-enhanced ultrasound imaging of tumor angiogenesis. Our results demonstrate that microbubbles conjugated to small peptide-targeting ligands provide imaging signals higher than those provided by a large antibody molecule.
Figures

References
-
- Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. - PubMed
-
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. - PubMed
-
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
