Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents
- PMID: 20150283
- PMCID: PMC2857905
- DOI: 10.2337/db09-1216
Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents
Abstract
Objective: The aim of this study was to examine differences between adolescents and adults in persistence of the benefits of intensive therapy 10 years after completion of the Diabetes Control and Complications Trial (DCCT).
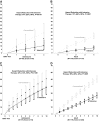
Research design and methods: During the Epidemiology of Diabetes Interventions and Complications (EDIC) study, progression of retinopathy from DCCT closeout to EDIC year 10 was evaluated in 1,055 adults and 156 adolescents.
Results: During 10 years of follow-up, HbA(1c) (A1C) was similar between original intensive (INT) and conventional (CON) groups and between former adolescents and adults. At EDIC year 10, adults in the former INT group continued to show slower progression of diabetic retinopathy than those in the CON group (adjusted hazard reduction 56%, P < 0.0001), whereas in adolescents this beneficial effect had disappeared (32%, P = 0.13). Seventy-nine percent of observed differences in the prolonged treatment effect between adults and adolescents at year 10 were explained by differences in mean A1C during DCCT between adolescents and adults (8.9 vs. 8.1%), particularly between INT adolescents and adults (8.1 vs. 7.2%).
Conclusions: Prior glycemic control during DCCT is vital for the persistence of the beneficial effects of INT therapy 10 years later. Lowering A1C to as close to normal as safely possible without severe hypoglycemia and starting as early as possible should be attempted for all subjects with type 1 diabetes. These results underscore the importance of maintaining A1C at target values for as long as possible because the benefits of former INT treatment wane over time if A1C levels rise.
Trial registration: ClinicalTrials.gov NCT00360815 NCT00360893.
Figures


References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 - PubMed
-
- White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WVDiabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Beneficial effects of intensive therapy of diabetes among adolescents: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–812 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

