Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression
- PMID: 20150284
- PMCID: PMC2857894
- DOI: 10.2337/db09-0807
Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression
Abstract
Objective: It is becoming apparent that there is a strong link between taste perception and energy homeostasis. Recent evidence implicates gut-related hormones in taste perception, including glucagon-like peptide 1 and vasoactive intestinal peptide (VIP). We used VIP knockout mice to investigate VIP's specific role in taste perception and connection to energy regulation.
Research design and methods: Body weight, food intake, and plasma levels of multiple energy-regulating hormones were measured and pancreatic morphology was determined. In addition, the immunocytochemical profile of taste cells and gustatory behavior were examined in wild-type and VIP knockout mice.
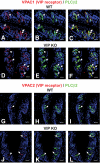
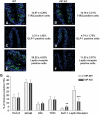
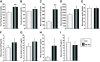
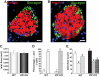
Results: VIP knockout mice demonstrate elevated plasma glucose, insulin, and leptin levels, with no islet beta-cell number/topography alteration. VIP and its receptors (VPAC1, VPAC2) were identified in type II taste cells of the taste bud, and VIP knockout mice exhibit enhanced taste preference to sweet tastants. VIP knockout mouse taste cells show a significant decrease in leptin receptor expression and elevated expression of glucagon-like peptide 1, which may explain sweet taste preference of VIP knockout mice.
Conclusions: This study suggests that the tongue can play a direct role in modulating energy intake to correct peripheral glycemic imbalances. In this way, we could view the tongue as a sensory mechanism that is bidirectionally regulated and thus forms a bridge between available foodstuffs and the intricate hormonal balance in the animal itself.
Figures

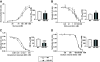

Similar articles
-
Vasoactive Intestinal Peptide (VIP) and its Receptors in Adipose Tissue: Implications for Cold Stress Adaptation.Cell Biochem Biophys. 2025 Jun;83(2):1963-1972. doi: 10.1007/s12013-024-01606-0. Epub 2024 Nov 17. Cell Biochem Biophys. 2025. PMID: 39550744
-
VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis.Cell Immunol. 2011;267(2):124-32. doi: 10.1016/j.cellimm.2011.01.001. Epub 2011 Jan 7. Cell Immunol. 2011. PMID: 21295288
-
Expression localisation and functional activity of pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal polypeptide and their receptors in mouse ovary.Reproduction. 2007 Aug;134(2):281-92. doi: 10.1530/REP-07-0051. Reproduction. 2007. PMID: 17660238
-
VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins.Br J Pharmacol. 2012 May;166(1):42-50. doi: 10.1111/j.1476-5381.2011.01676.x. Br J Pharmacol. 2012. PMID: 21951273 Free PMC article. Review.
-
PACAP and its receptors in cranial arteries and mast cells.J Headache Pain. 2018 Feb 20;19(1):16. doi: 10.1186/s10194-017-0822-2. J Headache Pain. 2018. PMID: 29460121 Free PMC article. Review.
Cited by
-
Altered hypothalamic protein expression in a rat model of Huntington's disease.PLoS One. 2012;7(10):e47240. doi: 10.1371/journal.pone.0047240. Epub 2012 Oct 18. PLoS One. 2012. PMID: 23094041 Free PMC article.
-
Higher TNF-α, IGF-1, and Leptin Levels are Found in Tasters than Non-Tasters.Front Endocrinol (Lausanne). 2014 Jul 29;5:125. doi: 10.3389/fendo.2014.00125. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25120532 Free PMC article.
-
Progressive and unconventional pharmacotherapeutic approaches to Alzheimer's disease therapy.Curr Alzheimer Res. 2012 Jan;9(1):1-4. doi: 10.2174/156720512799015082. Curr Alzheimer Res. 2012. PMID: 22329648 Free PMC article. No abstract available.
-
Endocrinology of Taste with Aging.Endocrinol Metab Clin North Am. 2023 Jun;52(2):295-315. doi: 10.1016/j.ecl.2022.10.002. Epub 2023 Feb 16. Endocrinol Metab Clin North Am. 2023. PMID: 36948781 Free PMC article. Review.
-
Metabolism and the circadian clock converge.Physiol Rev. 2013 Jan;93(1):107-35. doi: 10.1152/physrev.00016.2012. Physiol Rev. 2013. PMID: 23303907 Free PMC article. Review.
References
-
- Dulac C. The physiology of taste, vintage 2000. Cell 2000;100:607–610 - PubMed
-
- Yoshie S, Wakasugi C, Teraki Y, Fujita T. Fine structure of the taste bud in guinea pigs: I, cell characterization and innervation patterns. Arch Histol Cytol 1990;53:103–119 - PubMed
-
- Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 2001;440:97–108 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

