Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2(+) channels from secretory vesicles
- PMID: 20150285
- PMCID: PMC2857899
- DOI: 10.2337/db09-0791
Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2(+) channels from secretory vesicles
Abstract
Objective: The aim of the study was to elucidate the cellular mechanism underlying the suppression of glucose-induced insulin secretion in mice fed a high-fat diet (HFD) for 15 weeks.
Research design and methods: C57BL6J mice were fed a HFD or a normal diet (ND) for 3 or 15 weeks. Plasma insulin and glucose levels in vivo were assessed by intraperitoneal glucose tolerance test. Insulin secretion in vitro was studied using static incubations and a perfused pancreas preparation. Membrane currents, electrical activity, and exocytosis were examined by patch-clamp technique measurements. Intracellular calcium concentration ([Ca(2+)](i)) was measured by microfluorimetry. Total internal reflection fluorescence microscope (TIRFM) was used for optical imaging of exocytosis and submembrane depolarization-evoked [Ca(2+)](i). The functional data were complemented by analyses of histology and gene transcription.
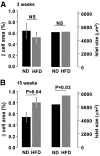
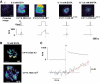
Results: After 15 weeks, but not 3 weeks, mice on HFD exhibited hyperglycemia and hypoinsulinemia. Pancreatic islet content and beta-cell area increased 2- and 1.5-fold, respectively. These changes correlated with a 20-50% reduction of glucose-induced insulin secretion (normalized to insulin content). The latter effect was not associated with impaired electrical activity or [Ca(2+)](i) signaling. Single-cell capacitance and TIRFM measurements of exocytosis revealed a selective suppression (>70%) of exocytosis elicited by short (50 ms) depolarization, whereas the responses to longer depolarizations were (500 ms) less affected. The loss of rapid exocytosis correlated with dispersion of Ca(2+) entry in HFD beta-cells. No changes in gene transcription of key exocytotic protein were observed.
Conclusions: HFD results in reduced insulin secretion by causing the functional dissociation of voltage-gated Ca(2+) entry from exocytosis. These observations suggest a novel explanation to the well-established link between obesity and diabetes.
Figures






References
-
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 - PubMed
-
- Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, Speizer FE. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501–513 - PubMed
-
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 - PubMed
-
- Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808 - PubMed
-
- Kaku K, Fiedorek FT, Jr, Province M, Permutt MA. Genetic analysis of glucose tolerance in inbred mouse strains: evidence for polygenic control. Diabetes 1988;37:707–713 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

