Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction
- PMID: 20150287
- PMCID: PMC2857893
- DOI: 10.2337/db09-1105
Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction
Abstract
Objective: Peripheral insulin resistance is linked to an increase in reactive oxygen species (ROS), leading in part to the production of reactive lipid aldehydes that modify the side chains of protein amino acids in a reaction termed protein carbonylation. The primary enzymatic method for lipid aldehyde detoxification is via glutathione S-transferase A4 (GSTA4) dependent glutathionylation. The objective of this study was to evaluate the expression of GSTA4 and the role(s) of protein carbonylation in adipocyte function.
Research design and methods: GSTA4-silenced 3T3-L1 adipocytes and GSTA4-null mice were evaluated for metabolic processes, mitochondrial function, and reactive oxygen species production. GSTA4 expression in human obesity was evaluated using microarray analysis.
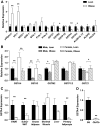
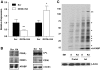
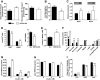
Results: GSTA4 expression is selectively downregulated in adipose tissue of obese insulin-resistant C57BL/6J mice and in human obesity-linked insulin resistance. Tumor necrosis factor-alpha treatment of 3T3-L1 adipocytes decreased GSTA4 expression, and silencing GSTA4 mRNA in cultured adipocytes resulted in increased protein carbonylation, increased mitochondrial ROS, dysfunctional state 3 respiration, and altered glucose transport and lipolysis. Mitochondrial function in adipocytes of lean or obese GSTA4-null mice was significantly compromised compared with wild-type controls and was accompanied by an increase in superoxide anion.
Conclusions: These results indicate that downregulation of GSTA4 in adipose tissue leads to increased protein carbonylation, ROS production, and mitochondrial dysfunction and may contribute to the development of insulin resistance and type 2 diabetes.
Figures





References
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–1555 - PubMed
-
- Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab 2003;285:E295–E302 - PubMed
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009February2 pii: 37048 doi: . [Epub ahead of print] - DOI - PMC - PubMed
-
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

