Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells
- PMID: 20150288
- PMCID: PMC2857896
- DOI: 10.2337/db09-0824
Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells
Abstract
Objective: Maternal adiponectin levels are reduced and placental nutrient transporters are upregulated in obesity and gestational diabetes mellitus; however, the effects of adiponectin on placental function are unknown. We hypothesized that adiponectin regulates placental amino acid transport.
Research design and methods: Human primary trophoblast cells were cultured and incubated with globular adiponectin (gAd) or full-length adiponectin (fAd) alone or in combination with insulin. System A and L amino acid transport and SNAT1, SNAT2, and SNAT4 isoform expression was measured. The activity of the AMP-activated protein kinase (AMPK), phosphatidylinositol 3 kinase-AKT, and peroxisome proliferator-activated receptor-alpha (PPARalpha) signaling pathways was determined.
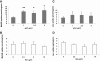
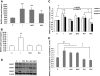
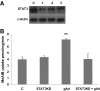
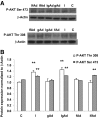
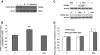
Results: In the absence of insulin, gAd stimulated AMPK Thr172 phosphorylation, SNAT2 protein expression, and system A activity. This effect appeared to be mediated by interleukin-6 release and signal transducer and activator of transcription 3 (STAT3) signaling because gAd failed to stimulate system A in cells in which STAT3 had been silenced using small interfering RNA. fAd alone had no effect on system A activity or SNAT expression. Insulin increased AKT and insulin receptor substrate 1 (IRS-1) phosphorylation, system A activity, and SNAT2 expression. When combined with insulin, gAd did not affect system A activity or SNAT expression. In contrast, fAd abolished insulin-stimulated AKT Thr308 and IRS-1 Tyr612 phosphorylation, system A activity, and SNAT2 expression. Furthermore, fAd increased PPARalpha expression and PPARalpha (Ser21) phosphorylation.
Conclusions: In contrast to the insulin-sensitizing actions of adiponectin in liver and muscle reported in the literature, fAd attenuates insulin signaling in primary human trophoblast cells. As a result, fAd inhibits insulin-stimulated amino acid transport, which may have important implications for placental nutrient transport and fetal growth in pregnancy complications associated with altered maternal adiponectin levels.
Figures
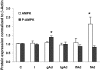

References
-
- Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstet Gynecol 2007;110:743–744 - PubMed
-
- Lam MH, Wong GY, Lao TT. Reappraisal of neonatal clavicular fracture: relationship between infant size and risk factors. J Reprod Med 2002;47:903–908 - PubMed
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 - PubMed
-
- Jansson T, Ekstrand Y, Björn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 2002;51:2214–2219 - PubMed
-
- Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol 1999;180:163–168 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

