MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes
- PMID: 20150292
- PMCID: PMC2858178
- DOI: 10.2337/dc09-1830
MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes
Abstract
Objective: Current state-of-the-art artificial pancreas systems are either based on traditional linear control theory or rely on mathematical models of glucose-insulin dynamics. Blood glucose control using these methods is limited due to the complexity of the biological system. The aim of this study was to describe the principles and clinical performance of the novel MD-Logic Artificial Pancreas (MDLAP) System.
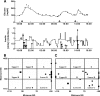
Research design and methods: The MDLAP applies fuzzy logic theory to imitate lines of reasoning of diabetes caregivers. It uses a combination of control-to-range and control-to-target strategies to automatically regulate individual glucose levels. Feasibility clinical studies were conducted in seven adults with type 1 diabetes (aged 19-30 years, mean diabetes duration 10 +/- 4 years, mean A1C 6.6 +/- 0.7%). All underwent 14 full, closed-loop control sessions of 8 h (fasting and meal challenge conditions) and 24 h.
Results: The mean peak postprandial (overall sessions) glucose level was 224 +/- 22 mg/dl. Postprandial glucose levels returned to <180 mg/dl within 2.6 +/- 0.6 h and remained stable in the normal range for at least 1 h. During 24-h closed-loop control, 73% of the sensor values ranged between 70 and 180 mg/dl, 27% were >180 mg/dl, and none were <70 mg/dl. There were no events of symptomatic hypoglycemia during any of the trials.
Conclusions: The MDLAP system is a promising tool for individualized glucose control in patients with type 1 diabetes. It is designed to minimize high glucose peaks while preventing hypoglycemia. Further studies are planned in the broad population under daily-life conditions.
Trial registration: ClinicalTrials.gov NCT00541515.
Figures
References
-
- Shalitin S, Phillip M: Closing the loop: combining insulin pumps and glucose sensors in children with type 1 diabetes mellitus. Pediatr Diabetes 2006; 7( Suppl. 4): 45– 49 - PubMed
-
- Steil G, Panteleon A, Rebrin K: Closed-loop insulin delivery: the path to physiological glucose control. Adv Drug Deliv Rev 2004; 56: 125– 144 - PubMed
-
- Parker R, Doyle Fr, Peppas N: A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng 1999; 46: 148– 157 - PubMed
-
- Hovorka R, Chassin L, Wilinska M, Canonico V, Akwi J, Federici M, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller H, Schaupp L, Bodenlenz M, Pieber T: Closing the loop: the adicol experience. Diabetes Technol Ther 2004; 6: 307– 318 - PubMed
-
- Hovorka R, Canonico V, Chassin L, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber T, Schaller H, Schaupp L, Vering T, Wilinska M: Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004; 25: 905– 920 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

