A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy
- PMID: 20150372
- PMCID: PMC2940554
- DOI: 10.1093/neuonc/nop015
A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy
Abstract
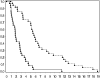
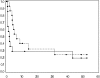
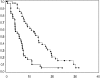
Patients with (a) recurrent malignant glioma (MG): glioblastoma (GBM) or recurrent anaplastic glioma (AG), and (b) nonprogressive (NP) GBM following radiation therapy (RT) were eligible. Primary objective for recurrent MG was progression-free survival at 6 months (PFS-6) and overall survival at 12 months for NP GBM post-RT. Secondary objectives for recurrent MGs were response, survival, assessment of toxicity, and pharmacokinetics (PKs). Treatment with enzyme-inducing antiepileptic drugs was not allowed. Patients received 150 mg/day erlotinib. Patients requiring surgery were treated 7 days prior to tumor removal for PK analysis and effects of erlotinib on epidermal growth factor receptor (EGFR) and intracellular signaling pathways. Ninety-six patients were evaluable (53 recurrent MG and 43 NP GBM); 5 patients were not evaluable for response. PFS-6 in recurrent GBM was 3% with a median PFS of 2 months; PFS-6 in recurrent AG was 27% with a median PFS of 2 months. Twelve-month survival was 57% in NP GBMs post-RT. Primary toxicity was dermatologic. The tissue-to-plasma ratio normalized to nanograms per gram dry weight for erlotinib and OSI-420 ranged from 25% to 44% and 30% to 59%, respectively, for pretreated surgical patients. No effect on EGFR or intratumoral signaling was seen. Patients with NP GBM post-RT who developed rash in cycle 1 had improved survival (P < .001). Single-agent activity of erlotinib is minimal for recurrent MGs and marginally beneficial following RT for NP GBM patients. Development of rash in cycle 1 correlates with survival in patients with NP GBM after RT.
Figures
References
-
- DeAngelis LM. Chemotherapy for brain tumors—a new beginning. N Engl J Med. 2005;352:1036–1038. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Nagane M, Coufal F, Lin H, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. - PubMed
-
- Rasheed BK, Wiltshire RN, Bigner SH, et al. Molecular pathogenesis of malignant gliomas. Curr Opin Oncol. 1999;11:162–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- M01-RR0865/RR/NCRR NIH HHS/United States
- M01-RR00079/RR/NCRR NIH HHS/United States
- U01CA62407-08/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- 5-U01CA62399-09/CA/NCI NIH HHS/United States
- M01-RR00056/RR/NCRR NIH HHS/United States
- CA62422/CA/NCI NIH HHS/United States
- U01CA62405/CA/NCI NIH HHS/United States
- CA62399/CA/NCI NIH HHS/United States
- CA62412/CA/NCI NIH HHS/United States
- U01 CA62399/CA/NCI NIH HHS/United States
- M01 RR003186/RR/NCRR NIH HHS/United States
- U01CA62421-08/CA/NCI NIH HHS/United States
- CA62426/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous