The functional role of Notch signaling in human gliomas
- PMID: 20150387
- PMCID: PMC2940575
- DOI: 10.1093/neuonc/nop022
The functional role of Notch signaling in human gliomas
Abstract
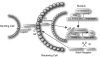
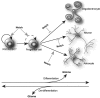
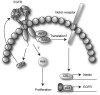
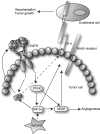
Gliomas are among the most devastating adult tumors for which there is currently no cure. The tumors are derived from brain glial tissue and comprise several diverse tumor forms and grades. Recent reports highlight the importance of cancer-initiating cells in the malignancy of gliomas. These cells have been referred to as brain cancer stem cells (bCSC), as they share similarities to normal neural stem cells in the brain. The Notch signaling pathway is involved in cell fate decisions throughout normal development and in stem cell proliferation and maintenance. The role of Notch in cancer is now firmly established, and recent data implicate a role for Notch signaling also in gliomas and bCSC. In this review, we explore the role of the Notch signaling pathway in gliomas with emphasis on its role in normal brain development and its interplay with pathways and processes that are characteristic of malignant gliomas.
Figures

Comment in
-
Tumor stem cells, notch, and the news.Neuro Oncol. 2010 Feb;12(2):115. doi: 10.1093/neuonc/nop055. Neuro Oncol. 2010. PMID: 20150377 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;10:987–996. - PubMed
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;19:7011–7021. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;18:5821–5828. - PubMed
-
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;5415:770–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

