Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK)
- PMID: 20150423
- PMCID: PMC2857020
- DOI: 10.1074/jbc.M109.090746
Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK)
Abstract
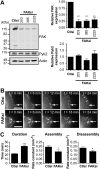
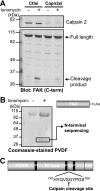
The coordinated and dynamic regulation of adhesions is required for cell migration. We demonstrated previously that limited proteolysis of talin1 by the calcium-dependent protease calpain 2 plays a critical role in adhesion disassembly in fibroblasts (Franco, S. J., Rodgers, M. A., Perrin, B. J., Han, J., Bennin, D. A., Critchley, D. R., and Huttenlocher, A. (2004) Nat. Cell Biol. 6, 977-983). However, little is known about the contribution of other calpain substrates to the regulation of adhesion dynamics. We now provide evidence that calpain 2-mediated proteolysis of focal adhesion kinase (FAK) regulates adhesion dynamics in motile cells. We mapped the preferred calpain cleavage site between the two C-terminal proline-rich regions after Ser-745, resulting in a C-terminal fragment similar in size to the FAK-related non-kinase (FRNK). We generated mutant FAK with a point mutation (V744G) that renders FAK resistant to calpain proteolysis but retains other biochemical properties of FAK. Using time-lapse microscopy, we show that the dynamics of green fluorescent protein-talin1 are impaired in FAK-deficient cells. Expression of wild-type but not calpain-resistant FAK rescues talin dynamics in FAK-deficient cells. Taken together, our findings suggest a novel role for calpain proteolysis of FAK in regulating adhesion dynamics in motile cells.
Figures




References
-
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 - PubMed
-
- Sieg D. J., Hauck C. R., Schlaepfer D. D. (1999) J. Cell Sci. 112, 2677–2691 - PubMed
-
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. (2004) Nat. Cell Biol. 6, 154–161 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

