Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity
- PMID: 20150427
- PMCID: PMC2865316
- DOI: 10.1074/jbc.M109.080390
Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity
Abstract
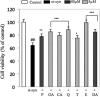
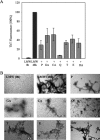
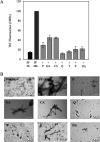
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease (AD). There is considerable consensus that the increased production and/or aggregation of alpha-synuclein (alpha-syn) plays a central role in the pathogenesis of PD and related synucleinopathies. Current therapeutic strategies for treating PD offer mainly transient symptomatic relief and aim at the restitution of dopamine levels to counterbalance the loss of dopaminergic neurons. Therefore, the identification and development of drug-like molecules that block alpha-synuclein aggregation and prevent the loss of dopaminergic neurons are desperately needed to treat or slow the progression of PD. Here, we show that entacapone and tolcapone are potent inhibitors of alpha-syn and beta-amyloid (Abeta) oligomerization and fibrillogenesis, and they also protect against extracellular toxicity induced by the aggregation of both proteins. Comparison of the anti-aggregation properties of entacapone and tolcapone with the effect of five other catechol-containing compounds, dopamine, pyrogallol, gallic acid, caffeic acid, and quercetin on the oligomerization and fibrillization of alpha-syn and Abeta, demonstrate that the catechol moiety is essential for the anti-amyloidogenic activity. Our findings present the first characterization of the anti-amyloidogenic properties of tolcapone and entacapone against both alpha-synuclein and Abeta42 and highlight the potential of this class of nitro-catechol compounds as anti-amyloidogenic agents. Their inhibitory properties, mode of action, and structural properties suggest that they constitute promising lead compounds for further optimization.
Figures

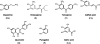




Similar articles
-
No change of brain extracellular catecholamine levels after acute catechol-O-methyltransferase inhibition: a microdialysis study in anaesthetized rats.Eur J Pharmacol. 1998 Sep 4;356(2-3):127-37. doi: 10.1016/s0014-2999(98)00524-x. Eur J Pharmacol. 1998. PMID: 9774242
-
Effects of peripheral and central catechol-O-methyltransferase inhibition on striatal extracellular levels of dopamine: a microdialysis study in freely moving rats.Parkinsonism Relat Disord. 2003 Jan;9(3):145-50. doi: 10.1016/s1353-8020(02)00016-0. Parkinsonism Relat Disord. 2003. PMID: 12573869
-
Synergistic inhibition of lung cancer cell lines by (-)-epigallocatechin-3-gallate in combination with clinically used nitrocatechol inhibitors of catechol-O-methyltransferase.Carcinogenesis. 2014 Feb;35(2):365-72. doi: 10.1093/carcin/bgt347. Epub 2013 Oct 22. Carcinogenesis. 2014. PMID: 24148818
-
General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase.Gen Pharmacol. 1994 Sep;25(5):813-24. doi: 10.1016/0306-3623(94)90082-5. Gen Pharmacol. 1994. PMID: 7835624 Review.
-
[Inhibition of catechol-O-methyltransferase. Optimizing dopaminergic therapy in idiopathic Parkinson syndrome with entacapone].Nervenarzt. 2000 Feb;71(2):78-83. doi: 10.1007/s001150050011. Nervenarzt. 2000. PMID: 10703007 Review. German.
Cited by
-
VPS35-Based Approach: A Potential Innovative Treatment in Parkinson's Disease.Front Neurol. 2019 Dec 17;10:1272. doi: 10.3389/fneur.2019.01272. eCollection 2019. Front Neurol. 2019. PMID: 31920908 Free PMC article. Review.
-
Novel therapeutic strategy for neurodegeneration by blocking Aβ seeding mediated aggregation in models of Alzheimer's disease.Neurobiol Dis. 2015 Feb;74:144-57. doi: 10.1016/j.nbd.2014.08.017. Epub 2014 Aug 28. Neurobiol Dis. 2015. PMID: 25173807 Free PMC article.
-
Monoaminergic and Histaminergic Strategies and Treatments in Brain Diseases.Front Neurosci. 2016 Nov 24;10:541. doi: 10.3389/fnins.2016.00541. eCollection 2016. Front Neurosci. 2016. PMID: 27932945 Free PMC article. Review.
-
Coconut (Cocos nucifera) Ethanolic Leaf Extract Reduces Amyloid-β (1-42) Aggregation and Paralysis Prevalence in Transgenic Caenorhabditis elegans Independently of Free Radical Scavenging and Acetylcholinesterase Inhibition.Biomedicines. 2017 Apr 21;5(2):17. doi: 10.3390/biomedicines5020017. Biomedicines. 2017. PMID: 28536360 Free PMC article.
-
Decoding crosstalk between neurotransmitters and α-synuclein in Parkinson's disease: pathogenesis and therapeutic implications.Ther Adv Neurol Disord. 2025 Jun 5;18:17562864251339895. doi: 10.1177/17562864251339895. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 40486190 Free PMC article. Review.
References
-
- Recchia A., Debetto P., Negro A., Guidolin D., Skaper S. D., Giusti P. (2004) FASEB J. 18, 617–626 - PubMed
-
- Sommer D. B., Stacy M. A. (2008) Expert Rev. Neurother. 8, 1829–1839 - PubMed
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 - PubMed
-
- Chung K. K., Dawson V. L., Dawson T. M. (2003) J. Neurol. 250, Suppl. 3, III15–24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

