Autophagy is a component of epithelial cell fate in obstructive uropathy
- PMID: 20150430
- PMCID: PMC2843468
- DOI: 10.2353/ajpath.2010.090345
Autophagy is a component of epithelial cell fate in obstructive uropathy
Abstract
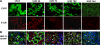
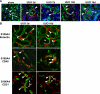
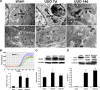
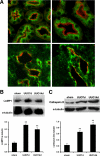
Epithelial cell fate and nephron loss in obstructive uropathy are not fully understood. We produced transgenic mice in which epithelial cells in the nephrons and collecting ducts were labeled with enhanced yellow fluorescent protein, and tracked the fate of these cells following unilateral ureteral obstruction (UUO). UUO led to a decrease in the number of enhanced yellow fluorescent protein-expressing cells and down-regulation of epithelial markers, E-cadherin, and hepatocyte nuclear factor-1beta. Following UUO, enhanced yellow fluorescent protein-positive cells were confined within the tubular basement membrane, were not found in the renal interstitium, and did not express alpha-smooth muscle actin or S100A4, markers of myofibroblasts and fibroblasts. Moreover, when proximal tubules were labeled with dextran before UUO, dextran-retaining cells did not migrate into the interstitium or express alpha-smooth muscle actin. These results indicate that UUO leads to tubular epithelial loss but does not cause epithelial-to-mesenchymal transition that has been shown by others to be responsible for nephron loss and interstitial fibrosis. For the first time, we found evidence of enhanced autophagy in obstructed tubules, including accumulation of autophagosomes, increased expression of Beclin 1, and increased conversion of microtubular-associated protein 1 light chain 3-I to -II. Increased autophagy may represent a mechanism of tubular survival or may contribute to excessive cell death and tubular atrophy after obstructive injury.
Figures




References
-
- Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the transplant registry: the 2006 annual report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant. 2007;11:366–373. - PubMed
-
- Chevalier RL. Pathogenesis of renal injury in obstructive uropathy. Curr Opin Pediatr. 2006;18:153–160. - PubMed
-
- Chevalier RL, Peters CA. Congenital urinary tract obstruction: proceedings of the State-Of-The-Art Strategic Planning Workshop-National Institutes of Health. Bethesda, Maryland, 11–12 March 2002. Pediatr Nephrol. 2003;18:576–606. - PubMed
-
- Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15:998–1007. - PubMed
-
- Potter E. Normal and abnormal development of the kidney. Chicago: Year Book Medical Publishers,; 1972:pp. 1–305.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

