Role of insulin-like growth factor binding protein 2 in lung adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3
- PMID: 20150439
- PMCID: PMC2843467
- DOI: 10.2353/ajpath.2010.090500
Role of insulin-like growth factor binding protein 2 in lung adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3
Abstract
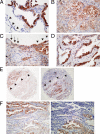
Insulin-like growth factor (IGF) signaling plays a pivotal role in cell proliferation and mitogenesis. Secreted IGF-binding proteins (IGFBPs) are important modulators of IGF bioavailability; however, their intracellular functions remain elusive. We sought to assess the antiapoptotic properties of intracellular IGFBP-2 in lung adenocarcinomas. IGFBP-2 overexpression resulted in a decrease in procaspase-3 expression; however, it did not influence the phosphorylation status of either IGF receptor or its downstream targets, including Akt and extracellular signal-regulated kinase. Apoptosis induced by camptothecin was significantly inhibited by IGFBP-2 overexpression in NCI-H522 cells. Conversely, selective knockdown of IGFBP-2 using small-interfering RNA resulted in an increase in procaspase-3 expression and sensitization to camptothecin-induced apoptosis in NCI-H522 cells. LY294002, an inhibitor of phosphatidyl-inositol 3-kinase, caused a decrease in IGFBP-2 levels and enhanced apoptosis in combination with camptothecin. Immunohistochemistry demonstrated that intracellular IGFBP-2 was highly expressed in lung adenocarcinomas compared with normal epithelium. Intracellular IGFBP-2 and procaspase-3 were expressed in a mutually exclusive manner. These findings suggest that intracellular IGFBP-2 regulates caspase-3 expression and contributes to the inhibitory effect on apoptosis independent of IGF. IGFBP-2, therefore, may offer a novel therapeutic target and serve as an antiapoptotic biomarker for lung adenocarcinoma.
Figures





References
-
- Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, Rossjohn J, Price JT. Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with alphavbeta3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res. 2004;64:977–984. - PubMed
-
- Wang GK, Hu L, Fuller GN, Zhang W. An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J Biol Chem. 2006;281:14085–14091. - PubMed
-
- Russo VC, Bach LA, Fosang AJ, Baker NL, Werther GA. Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb. Endocrinology. 1997;138:4858–4867. - PubMed
-
- Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. - PubMed
-
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

