In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation
- PMID: 20150509
- PMCID: PMC2840160
- DOI: 10.1073/pnas.0915156107
In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation
Abstract
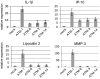
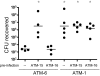
Type VI secretion systems (T6SSs) have recently been recognized as potential virulence determinants of many Gram-negative bacterial pathogens. Although mechanistic studies are lacking, T6SS-dependent phenotypes can be observed in various animal models of infection. Presumably translocation of T6SS effectors into target cells is involved in virulence, but few such effectors have been identified. A hallmark of T6SS function is the in vitro secretion of Hcp and VgrG proteins, which are thought to form part of an extracellular secretion apparatus. One well-characterized effector domain is the C-terminal actin cross-linking domain (ACD) of the VgrG-1 protein, constitutively secreted by the T6SS of Vibrio cholerae strain V52. Previous work indicated that translocation of VgrG-1 occurred only after endocytic uptake of bacteria into host cells. VgrG-1-induced actin cross-linking impaired phagocytic activity of host cells, eventually causing cell death. To determine whether V. cholerae T6SS is functional during animal infection, derivatives of V52 were used to infect infant mice. In this infection model a diarrheal response occurred, and actin cross-linking could be detected. These host responses were dependent on a functional T6SS and on the ACD of VgrG-1. Gene expression and histologic studies showed innate immune activation and immune cell infiltration in the intestinal lumen. The T6SS-dependent inflammatory response was also associated with increased recovery of V. cholerae from the intestine. We conclude that the T6SS of V52 induces an inflammatory diarrhea that facilitates replication of V. cholerae within the intestine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe. 2007;1:95–107. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

