Ketosteroid isomerase provides further support for the idea that enzymes work by electrostatic preorganization
- PMID: 20150513
- PMCID: PMC2840163
- DOI: 10.1073/pnas.0914579107
Ketosteroid isomerase provides further support for the idea that enzymes work by electrostatic preorganization
Abstract
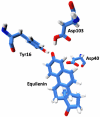
One of the best systems for exploring the origin of enzyme catalysis has been the reaction of ketosteroid isomerase (KSI). Studies of the binding of phenolates to KSI have been taken as proof that the electrostatic preorganization effect only makes a minor contribution to the binding of the real, multiring, transition state (TS). However, our simulation study has determined that the difference between the phenolates and the TS arises from the fact that the nonpolar state of the phenolate can rotate freely relative to the oxyanion hole and thus loses the preorganization contribution. A recent study explored the reactivity of both small and multiring systems and concluded that their similar reactivity contradicts our preorganization idea. Herein, we establish that the available experiments in fact provide what is perhaps the best proof and clarification of the preorganization idea and its crucial role in enzyme catalysis. First, we analyze the binding energy and the pK(a) of equilenin and identify direct experimental evidence for our prediction about the differential electrostatic stabilization of the large TS and the small phenolates. Subsequently, we show that the similar reactivity of the small and large systems is also due to an electrostatic preorganization effect but that this effect only appears in the intermediate state because the TS is not free to rotate. This establishes the electrostatic origin of enzyme catalysis. We also clarify the crucial importance of having a well-defined physical concept when examining catalytic effects and the need for quantitative tools for analyzing such effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
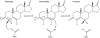

Similar articles
-
Electrostatic contributions to binding of transition state analogues can be very different from the corresponding contributions to catalysis: phenolates binding to the oxyanion hole of ketosteroid isomerase.Biochemistry. 2007 Feb 13;46(6):1466-76. doi: 10.1021/bi061752u. Biochemistry. 2007. PMID: 17279612
-
A Preorganized Electric Field Leads to Minimal Geometrical Reorientation in the Catalytic Reaction of Ketosteroid Isomerase.J Am Chem Soc. 2020 Jun 3;142(22):9993-9998. doi: 10.1021/jacs.0c00383. Epub 2020 May 19. J Am Chem Soc. 2020. PMID: 32378409 Free PMC article.
-
Testing electrostatic complementarity in enzyme catalysis: hydrogen bonding in the ketosteroid isomerase oxyanion hole.PLoS Biol. 2006 Apr;4(4):e99. doi: 10.1371/journal.pbio.0040099. Epub 2006 Mar 28. PLoS Biol. 2006. PMID: 16602823 Free PMC article.
-
Electric Fields and Enzyme Catalysis.Annu Rev Biochem. 2017 Jun 20;86:387-415. doi: 10.1146/annurev-biochem-061516-044432. Epub 2017 Mar 24. Annu Rev Biochem. 2017. PMID: 28375745 Free PMC article. Review.
-
Structure and enzymology of Delta5-3-ketosteroid isomerase.Curr Opin Struct Biol. 2001 Dec;11(6):674-8. doi: 10.1016/s0959-440x(01)00268-8. Curr Opin Struct Biol. 2001. PMID: 11751047 Review.
Cited by
-
Catalytic Fields as a Tool to Analyze Enzyme Reaction Mechanism Variants and Reaction Steps.J Phys Chem B. 2021 Oct 28;125(42):11606-11616. doi: 10.1021/acs.jpcb.1c05256. Epub 2021 Oct 14. J Phys Chem B. 2021. PMID: 34648705 Free PMC article.
-
Computational protein engineering: bridging the gap between rational design and laboratory evolution.Int J Mol Sci. 2012 Sep 28;13(10):12428-60. doi: 10.3390/ijms131012428. Int J Mol Sci. 2012. PMID: 23202907 Free PMC article. Review.
-
Machine Learning Identifies Chemical Characteristics That Promote Enzyme Catalysis.J Am Chem Soc. 2019 Mar 6;141(9):4108-4118. doi: 10.1021/jacs.8b13879. Epub 2019 Feb 21. J Am Chem Soc. 2019. PMID: 30761897 Free PMC article.
-
Quantum mechanical modeling: a tool for the understanding of enzyme reactions.Biomolecules. 2013 Sep 23;3(3):662-702. doi: 10.3390/biom3030662. Biomolecules. 2013. PMID: 24970187 Free PMC article.
-
Local Electric Fields as a Natural Switch of Heme-Iron Protein Reactivity.ACS Catal. 2021 Jun 4;11(11):6534-6546. doi: 10.1021/acscatal.1c00687. Epub 2021 May 19. ACS Catal. 2021. PMID: 34413991 Free PMC article.
References
-
- Warshel A, et al. Electrostatic basis for enzyme catalysis. Chem Rev. 2006;106:3210–3235. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

