SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweetpotato (Ipomoea batatas)
- PMID: 20150515
- PMCID: PMC2837253
- DOI: 10.1093/jxb/erp399
SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweetpotato (Ipomoea batatas)
Abstract
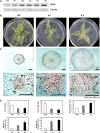
A sweetpotato (Ipomoea batatas cv. 'Jinhongmi') MADS-box protein cDNA (SRD1) has been isolated from an early stage storage root cDNA library. The role of the SRD1 gene in the formation of the storage root in sweetpotato was investigated by an expression pattern analysis and characterization of SRD1-overexpressing (ox) transgenic sweetpotato plants. Transcripts of SRD1 were detected only in root tissues, with the fibrous root having low levels of the transcript and the young storage root showing relatively higher transcript levels. SRD1 mRNA was mainly found in the actively dividing cells, including the vascular and cambium cells of the young storage root. The transcript level of SRD1 in the fibrous roots increased in response to 1000 muM indole-3-acetic acid (IAA) applied exogenously. During the early stage of storage root development, the endogenous IAA content and SRD1 transcript level increased concomitantly, suggesting an involvement of SRD1 during the early stage of the auxin-dependent development of the storage root. SRD1-ox sweetpotato plants cultured in vitro produced thicker and shorter fibrous roots than wild-type plants. The metaxylem and cambium cells of the fibrous roots of SRD1-ox plants showed markedly enhanced proliferation, resulting in the fibrous roots of these plants showing an earlier thickening growth than those of wild-type plants. Taken together, these results demonstrate that SRD1 plays a role in the formation of storage roots by activating the proliferation of cambium and metaxylem cells to induce the initial thickening growth of storage roots in an auxin-dependent manner.
Figures






References
-
- Akita S, Yamamoto F, Ono M, Kusuhara M, Kobayashi H, Ikemoto S. Studies on the small tuber set method in sweet potato cultivation. Bulletin of the Chugoku National Agricultural Experiment Station. 1962;8:75–128. (in Japanese with English summary)
-
- Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF. MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. The Plant Journal. 2000;24:457–466. - PubMed
-
- Boonplod N. 2005. Changes in the concentration of particular hormones and carbohydrates in apple shoots after ‘bending’ respectively chemical treatments and relationship to flower induction process. PhD thesis, University of Hohenheim, Stuttgart, Germany.
-
- Carmona MJ, Ortega N, Garcia-Maroto F. Isolation and molecular characterization of a new vegetative MADS-box gene from Solanum tuberosum L. Planta. 1998;207:181–188. - PubMed
-
- Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Current Biology. 1996;6:325–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

