Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4
- PMID: 20150540
- PMCID: PMC2865747
- DOI: 10.1681/ASN.2009060597
Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4
Abstract
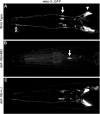
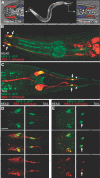
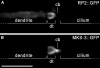
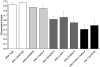
Cilia dysfunction contributes to renal cyst formation in multiple human syndromes including nephronophthisis (NPHP), Meckel-Gruber syndrome (MKS), Joubert syndrome (JBTS), and Bardet-Beidl syndrome (BBS). Although genetically heterogeneous, these diseases share several loci that affect cilia and/or basal body proteins, but the functions and interactions of these gene products are incompletely understood. Here, we report that the ciliated sensory neurons (CSNs) of C. elegans express the putative transmembrane protein MKS-3, which localized to the distal end of their dendrites and to the cilium base but not to the cilium itself. Localization of MKS-3 and other known MKS and NPHP proteins partially overlapped. By analyzing mks-3 mutants, we found that ciliogenesis did not require MKS-3; instead, cilia elongated and cilia-mediated chemoreception was abnormal. Genetic analysis indicated that mks-3 functions in a pathway with other mks genes. Furthermore, mks-1 and mks-3 genetically interacted with a separate pathway (involving nphp-1 and nphp-4) to influence proper positioning, orientation, and formation of cilia. Combined disruption of nphp and mks pathways had cell nonautonomous effects on C. elegans sensilla. Taken together, these data demonstrate the importance of mutational load on the presentation and severity of ciliopathies and expand the understanding of the interactions between ciliopathy genes.
Figures




Comment in
-
Synergistic interaction between ciliary genes reflects the importance of mutational load in ciliopathies.J Am Soc Nephrol. 2010 May;21(5):724-6. doi: 10.1681/ASN.2010030301. Epub 2010 Apr 15. J Am Soc Nephrol. 2010. PMID: 20395369 No abstract available.
Similar articles
-
Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins.J Cell Sci. 2012 Jun 1;125(Pt 11):2592-603. doi: 10.1242/jcs.095539. Epub 2012 Mar 5. J Cell Sci. 2012. PMID: 22393243 Free PMC article.
-
MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis.J Cell Biol. 2011 Mar 21;192(6):1023-41. doi: 10.1083/jcb.201012116. J Cell Biol. 2011. PMID: 21422230 Free PMC article.
-
Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis.Mol Biol Cell. 2008 May;19(5):2154-68. doi: 10.1091/mbc.e07-10-1070. Epub 2008 Mar 12. Mol Biol Cell. 2008. PMID: 18337471 Free PMC article.
-
Ciliopathies and the Kidney: A Review.Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review.
-
The ciliary transitional zone and nephrocystins.Differentiation. 2012 Feb;83(2):S91-6. doi: 10.1016/j.diff.2011.11.006. Epub 2011 Dec 12. Differentiation. 2012. PMID: 22169048 Review.
Cited by
-
Cleavage of the Meckel-Gruber syndrome protein TMEM67 by ADAMTS9 uncouples Wnt signaling and ciliogenesis.Nat Commun. 2025 May 28;16(1):4946. doi: 10.1038/s41467-025-60294-3. Nat Commun. 2025. PMID: 40436881 Free PMC article.
-
Clock genes rescue nphp mutations in zebrafish.Hum Mol Genet. 2022 Dec 16;31(24):4143-4158. doi: 10.1093/hmg/ddac160. Hum Mol Genet. 2022. PMID: 35861640 Free PMC article.
-
Measurement of cytoplasmic and cilioplasmic calcium in a single living cell.Methods Cell Biol. 2019;153:25-42. doi: 10.1016/bs.mcb.2019.03.015. Epub 2019 Apr 17. Methods Cell Biol. 2019. PMID: 31395382 Free PMC article.
-
Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport.Dev Biol. 2011 Sep 1;357(1):235-47. doi: 10.1016/j.ydbio.2011.06.028. Epub 2011 Jun 27. Dev Biol. 2011. PMID: 21740898 Free PMC article.
-
Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease.Organogenesis. 2014 Jan 1;10(1):126-37. doi: 10.4161/org.28830. Epub 2014 Apr 14. Organogenesis. 2014. PMID: 24732235 Free PMC article. Review.
References
-
- Alexiev BA, Lin X, Sun C. C., Brenner DS: Meckel-Gruber syndrome: Pathologic Manifestations, Minimal diagnostic criteria, and differential diagnosis. Arch Pathol Lab Med 130: 1236–1238, 2006. - PubMed
-
- Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, Esculpavit C, Toutain A, Moraine C, Parent P, Marcorelles P, Dauge MC, Roume J, Le Merrer M, Meiner V, Meir K, Menez F, Beaufrere AM, Francannet C, Tantau J, Sinico M, Dumez Y, MacDonald F, Munnich A, Lyonnet S, Gubler MC, Genin E, Johnson CA, Vekemans M, Encha-Razavi F., Attie-Bitach T.: Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet 81: 170–179, 2007. - PMC - PubMed
-
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S: The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39: 875–881, 2007. - PubMed
-
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone V H, II, Harris PC, Johnson CA: The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet 38: 191–196, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

