Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat
- PMID: 20150564
- PMCID: PMC2867532
- DOI: 10.1152/japplphysiol.01285.2009
Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat
Abstract
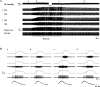
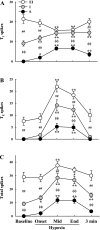
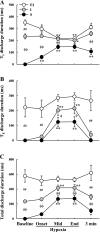
Respiratory-related discharge in the hypoglossal (XII) nerve is composed of preinspiratory (pre-I) and inspiratory (I) activity. Our first purpose was to test the hypothesis that hypoxia-induced plasticity in XII motor output is differentially expressed in pre-I vs. I XII bursting. Short-term potentiation (STP) of XII motor output was induced in urethane-anesthetized, vagotomized, and ventilated rats by exposure to isocapnic hypoxia (PaO2 of approximately 35 Torr). Both pre-I and I XII discharge abruptly increased at beginning of hypoxia (i.e., acute hypoxic response), and the relative increase in amplitude was much greater for pre-I (507+/-46% baseline) vs. I bursting (257+/-16% baseline; P<0.01). In addition, STP was expressed in I but not pre-I bursting following hypoxia. Specifically, I activity remained elevated following termination of hypoxia but pre-I bursting abruptly returned to prehypoxia levels. Our second purpose was to test the hypothesis that STP of I XII activity results from recruitment of inactive or "silent" XII motoneurons (MNs) vs. rate coding of active MNs. Single fiber recordings were used to classify XII MNs as I, expiratory-inspiratory, or silent based on baseline discharge patterns. STP of I XII activity following hypoxia was associated with increased discharge frequency in active I and silent MNs but not expiratory-inspiratory MNs. We conclude that the expression of respiratory plasticity is differentially regulated between pre-I and I XII activity. In addition, both recruitment of silent MNs and rate coding of active I MNs contribute to increases in XII motor output following hypoxia.
Figures







Similar articles
-
Hypoxia-induced short-term potentiation of respiratory-modulated facial motor output in the rat.Respir Physiol Neurobiol. 2010 Aug 31;173(1):107-11. doi: 10.1016/j.resp.2010.06.015. Epub 2010 Jul 1. Respir Physiol Neurobiol. 2010. PMID: 20601212 Free PMC article.
-
Vagal modulation of pre-inspiratory activity in hypoglossal discharge in the decerebrate rat.Respir Physiol Neurobiol. 2015 Aug 15;215:47-50. doi: 10.1016/j.resp.2015.05.002. Epub 2015 May 12. Respir Physiol Neurobiol. 2015. PMID: 25979456
-
Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats.J Neurophysiol. 2009 Oct;102(4):2184-93. doi: 10.1152/jn.00399.2009. Epub 2009 Aug 5. J Neurophysiol. 2009. PMID: 19657076 Free PMC article.
-
Retracted: Control of hypoglossal pre-inspiratory discharge.Exp Physiol. 2020 Aug;105(8):1232-1255. doi: 10.1113/EP087329. Epub 2020 Jul 16. Exp Physiol. 2020. Retraction in: Exp Physiol. 2020 Nov;105(11):1972. doi: 10.1113/EP089093. PMID: 32539192 Retracted. Review.
-
Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms.Arch Ital Biol. 2011 Dec 1;149(4):426-53. doi: 10.4449/aib.v149i4.1271. Arch Ital Biol. 2011. PMID: 22205594 Review.
Cited by
-
Pulmonary C-fiber activation attenuates respiratory-related tongue movements.J Appl Physiol (1985). 2012 Nov;113(9):1369-76. doi: 10.1152/japplphysiol.00031.2012. Epub 2012 Aug 30. J Appl Physiol (1985). 2012. PMID: 22936725 Free PMC article.
-
Pharmacological modulation of hypoxia-induced respiratory neuroplasticity.Respir Physiol Neurobiol. 2018 Oct;256:4-14. doi: 10.1016/j.resp.2017.11.008. Epub 2017 Nov 29. Respir Physiol Neurobiol. 2018. PMID: 29197629 Free PMC article. Review.
-
Hypoxia-induced short-term potentiation of respiratory-modulated facial motor output in the rat.Respir Physiol Neurobiol. 2010 Aug 31;173(1):107-11. doi: 10.1016/j.resp.2010.06.015. Epub 2010 Jul 1. Respir Physiol Neurobiol. 2010. PMID: 20601212 Free PMC article.
-
Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson's Disease.Cells. 2021 Mar 2;10(3):531. doi: 10.3390/cells10030531. Cells. 2021. PMID: 33801475 Free PMC article.
-
Activities of human genioglossus motor units.Respir Physiol Neurobiol. 2011 Oct 15;179(1):14-22. doi: 10.1016/j.resp.2011.04.018. Epub 2011 Apr 22. Respir Physiol Neurobiol. 2011. PMID: 21558022 Free PMC article. Review.
References
-
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 - PubMed
-
- Bach KB, Mitchell GS. Effects of phrenicotomy and exercise on hypoxia-induced changes in phrenic motor output. J Appl Physiol 89: 1884–1891, 2000 - PubMed
-
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

