Polymer gel dosimetry
- PMID: 20150687
- PMCID: PMC3031873
- DOI: 10.1088/0031-9155/55/5/R01
Polymer gel dosimetry
Abstract
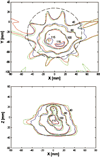
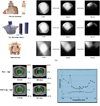
Polymer gel dosimeters are fabricated from radiation sensitive chemicals which, upon irradiation, polymerize as a function of the absorbed radiation dose. These gel dosimeters, with the capacity to uniquely record the radiation dose distribution in three-dimensions (3D), have specific advantages when compared to one-dimensional dosimeters, such as ion chambers, and two-dimensional dosimeters, such as film. These advantages are particularly significant in dosimetry situations where steep dose gradients exist such as in intensity-modulated radiation therapy (IMRT) and stereotactic radiosurgery. Polymer gel dosimeters also have specific advantages for brachytherapy dosimetry. Potential dosimetry applications include those for low-energy x-rays, high-linear energy transfer (LET) and proton therapy, radionuclide and boron capture neutron therapy dosimetries. These 3D dosimeters are radiologically soft-tissue equivalent with properties that may be modified depending on the application. The 3D radiation dose distribution in polymer gel dosimeters may be imaged using magnetic resonance imaging (MRI), optical-computerized tomography (optical-CT), x-ray CT or ultrasound. The fundamental science underpinning polymer gel dosimetry is reviewed along with the various evaluation techniques. Clinical dosimetry applications of polymer gel dosimetry are also presented.
Figures














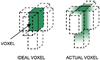






References
-
- Adamovics J, Maryanski MJ. Characterisation of PRESAGE: a new 3-D radiochromic solid polymer dosemeter for ionising radiation. Radiat. Prot. Dosim. 2006;120:107–112. - PubMed
-
- Alexander P, Charlesby A, Ross M. The degradation of solid polymethylmethacrylate by ionizing radiations. Proc. R. Soc. A. 1954;223:392.
-
- Amin MN, Horsfield MA, Bonnett DE, Dunn MJ, Poulton M, Harding PF. A comparison of polyacrylamide gels and radiochromic film for source measurements in intravascular brachytherapy. Br. J. Radiol. 2003;76:824–831. - PubMed
-
- Andrews HL, Murphy RE, LeBrun EJ. Gel dosimeter for depth dose measurements. Rev. Sci. Instrum. 1957;28:329–332.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
