Neutralization of Clostridium difficile toxin A using antibody combinations
- PMID: 20150758
- PMCID: PMC2840238
- DOI: 10.4161/mabs.2.2.11220
Neutralization of Clostridium difficile toxin A using antibody combinations
Abstract
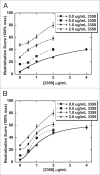
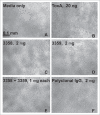
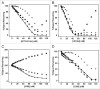
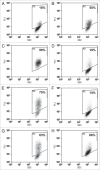
The pathogenicity of Clostridium difficile (C. difficile) is mediated by the release of two toxins, A and B. Both toxins contain large clusters of repeats known as cell wall binding (CWB) domains responsible for binding epithelial cell surfaces. Several murine monoclonal antibodies were generated against the CWB domain of toxin A and screened for their ability to neutralize the toxin individually and in combination. Three antibodies capable of neutralizing toxin A all recognized multiple sites on toxin A, suggesting that the extent of surface coverage may contribute to neutralization. Combination of two noncompeting antibodies, denoted 3358 and 3359, enhanced toxin A neutralization over saturating levels of single antibodies. Antibody 3358 increased the level of detectable CWB domain on the surface of cells, while 3359 inhibited CWB domain cell surface association. These results suggest that antibody combinations that cover a broader epitope space on the CWB repeat domains of toxin A (and potentially toxin B) and utilize multiple mechanisms to reduce toxin internalization may provide enhanced protection against C. difficile-associated diarrhea.
Figures
References
-
- Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am. 2001;30:753–777. - PubMed
-
- Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile-associated diarrhea. Arch Int Med. 2001;161:525–533. - PubMed
-
- Pothoulakis C, LaMont JT. Microbes and microbial toxins: paradigms for microbial mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280:178–183. - PubMed
-
- Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North Americ and Europe. Clin Microbiol Infect. 2008;12:2–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
