Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival
- PMID: 20150892
- PMCID: PMC2845279
- DOI: 10.1038/emboj.2010.7
Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival
Abstract
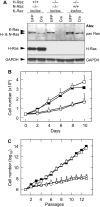
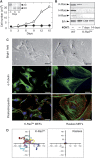
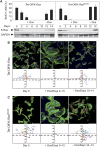
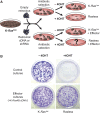
We have used mouse embryonic fibroblasts (MEFs) devoid of Ras proteins to illustrate that they are essential for proliferation and migration, but not for survival, at least in these cells. These properties are unique to the Ras subfamily of proteins because ectopic expression of other Ras-like small GTPases, even when constitutively active, could not compensate for the absence of Ras proteins. Only constitutive activation of components of the Raf/Mek/Erk pathway was sufficient to sustain normal proliferation and migration of MEFs devoid of Ras proteins. Activation of the phosphatidylinositol 3-kinase (PI3K)/PTEN/Akt and Ral guanine exchange factor (RalGEF)/Ral pathways, either alone or in combination, failed to induce proliferation or migration of Rasless cells, although they cooperated with Raf/Mek/Erk signalling to reproduce the full response mediated by Ras signalling. In contrast to current hypotheses, Ras signalling did not induce proliferation by inducing expression of D-type Cyclins. Rasless MEFs had normal levels of Cyclin D1/Cdk4 and Cyclin E/Cdk2. However, these complexes were inactive. Inactivation of the pocket proteins or knock down of pRb relieved MEFs from their dependence on Ras signalling to proliferate.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

