Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development
- PMID: 20150895
- PMCID: PMC2857457
- DOI: 10.1038/emboj.2010.10
Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development
Abstract
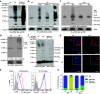
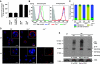
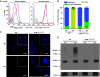
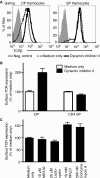
Expression of the T-cell receptor (TCR):CD3 complex is tightly regulated during T-cell development. The mechanism and physiological role of this regulation are unclear. Here, we show that the TCR:CD3 complex is constitutively ubiquitylated in immature double positive (DP) thymocytes, but not mature single positive (SP) thymocytes or splenic T cells. This steady state, tonic CD3 monoubiquitylation is mediated by the CD3varepsilon proline-rich sequence, Lck, c-Cbl, and SLAP, which collectively trigger the dynamin-dependent downmodulation, lysosomal sequestration and degradation of surface TCR:CD3 complexes. Blocking this tonic ubiquitylation by mutating all the lysines in the CD3 cytoplasmic tails significantly upregulates TCR levels on DP thymocytes. Mimicking monoubiquitylation by expression of a CD3zeta-monoubiquitin (monoUb) fusion molecule significantly reduces TCR levels on immature thymocytes. Moreover, modulating CD3 ubiquitylation alters immunological synapse (IS) formation and Erk phosphorylation, thereby shifting the signalling threshold for positive and negative selection, and regulatory T-cell development. Thus, tonic TCR:CD3 ubiquitylation results in precise regulation of TCR expression on immature T cells, which is required to maintain the fidelity of T-cell development.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Reduced Cbl phosphorylation and degradation of the zeta-chain of the T-cell receptor/CD3 complex in T cells with low Lck levels.Eur J Immunol. 2008 Sep;38(9):2557-63. doi: 10.1002/eji.200737837. Eur J Immunol. 2008. PMID: 18792408
-
Regulation of T cell receptor (TCR) beta gene expression by CD3 complex signaling in immature thymocytes: implications for TCRbeta allelic exclusion.Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3882-7. doi: 10.1073/pnas.96.7.3882. Proc Natl Acad Sci U S A. 1999. PMID: 10097132 Free PMC article.
-
Expression of fully assembled TCR-CD3 complex on double positive thymocytes: synergistic role for the PRS and ER retention motifs in the intra-cytoplasmic tail of CD3epsilon.Int Immunol. 2009 Dec;21(12):1317-27. doi: 10.1093/intimm/dxp098. Epub 2009 Oct 9. Int Immunol. 2009. PMID: 19819936
-
Selected signalling proteins recruited to the T-cell receptor-CD3 complex.Immunology. 2018 Jan;153(1):42-50. doi: 10.1111/imm.12809. Epub 2017 Sep 5. Immunology. 2018. PMID: 28771705 Free PMC article. Review.
-
TCR trafficking in resting and stimulated T cells.Crit Rev Immunol. 2004;24(1):67-86. doi: 10.1615/critrevimmunol.v24.i1.30. Crit Rev Immunol. 2004. PMID: 14995914 Review.
Cited by
-
CD3e-immunotoxin spares CD62Llo Tregs and reshapes organ-specific T-cell composition by preferentially depleting CD3ehi T cells.Front Immunol. 2022 Oct 26;13:1011190. doi: 10.3389/fimmu.2022.1011190. eCollection 2022. Front Immunol. 2022. PMID: 36389741 Free PMC article.
-
Membrane association of the CD3ε signaling domain is required for optimal T cell development and function.J Immunol. 2014 Jul 1;193(1):258-67. doi: 10.4049/jimmunol.1400322. Epub 2014 Jun 4. J Immunol. 2014. PMID: 24899501 Free PMC article.
-
The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression.Int J Mol Sci. 2023 Sep 25;24(19):14505. doi: 10.3390/ijms241914505. Int J Mol Sci. 2023. PMID: 37833951 Free PMC article.
-
Usp12 stabilizes the T-cell receptor complex at the cell surface during signaling.Proc Natl Acad Sci U S A. 2016 Feb 9;113(6):E705-14. doi: 10.1073/pnas.1521763113. Epub 2016 Jan 25. Proc Natl Acad Sci U S A. 2016. PMID: 26811477 Free PMC article.
-
The Emerging and Diverse Roles of Src-Like Adaptor Proteins in Health and Disease.Mediators Inflamm. 2015;2015:952536. doi: 10.1155/2015/952536. Epub 2015 Aug 3. Mediators Inflamm. 2015. PMID: 26339145 Free PMC article. Review.
References
-
- Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M (2002) Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296: 2036–2040 - PubMed
-
- Aivazian D, Stern LJ (2000) Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol 7: 1023–1026 - PubMed
-
- Bluestone JA, Pardoll D, Sharrow SO, Fowlkes BJ (1987) Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature 326: 82–84 - PubMed
-
- Brodeur JF, Li S, Martins MS, Larose L, Dave VP (2009) Critical and multiple roles for the CD3epsilon intracytoplasmic tail in double negative to double positive thymocyte differentiation. J Immunol 182: 4844–4853 - PubMed
-
- Cadwell K, Coscoy L (2005) Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309: 127–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

