The Natural Selection of Herpesviruses and Virus-Specific NK Cell Receptors
- PMID: 20151027
- PMCID: PMC2819288
- DOI: 10.3390/v1030362
The Natural Selection of Herpesviruses and Virus-Specific NK Cell Receptors
Abstract
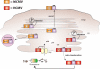
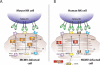
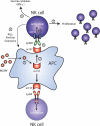
During the co-evolution of cytomegalovirus (CMV) and natural killer (NK) cells, each has evolved specific tactics in an attempt to prevail. CMV has evolved multiple immune evasion mechanisms to avoid detection by NK cells and other immune cells, leading to chronic infection. Meanwhile, the host has evolved virus-specific receptors to counter these evasion strategies. The natural selection of viral genes and host receptors allows us to observe a unique molecular example of "survival of the fittest", as virus and immune cells try to out-maneuver one another or for the virus to achieve détente for optimal dissemination in the population.
Figures



Similar articles
-
Hematopoietic cell-mediated dissemination of murine cytomegalovirus is regulated by NK cells and immune evasion.PLoS Pathog. 2021 Jan 28;17(1):e1009255. doi: 10.1371/journal.ppat.1009255. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33508041 Free PMC article.
-
All is fair in virus-host interactions: NK cells and cytomegalovirus.Trends Mol Med. 2011 Nov;17(11):677-85. doi: 10.1016/j.molmed.2011.07.003. Epub 2011 Aug 17. Trends Mol Med. 2011. PMID: 21852192 Free PMC article.
-
How the virus outsmarts the host: function and structure of cytomegalovirus MHC-I-like molecules in the evasion of natural killer cell surveillance.J Biomed Biotechnol. 2011;2011:724607. doi: 10.1155/2011/724607. Epub 2011 Jun 30. J Biomed Biotechnol. 2011. PMID: 21765638 Free PMC article. Review.
-
Deciphering the Immunological Phenomenon of Adaptive Natural Killer (NK) Cells and Cytomegalovirus (CMV).Int J Mol Sci. 2020 Nov 23;21(22):8864. doi: 10.3390/ijms21228864. Int J Mol Sci. 2020. PMID: 33238550 Free PMC article. Review.
-
DNAM-1 Activating Receptor and Its Ligands: How Do Viruses Affect the NK Cell-Mediated Immune Surveillance during the Various Phases of Infection?Int J Mol Sci. 2019 Jul 30;20(15):3715. doi: 10.3390/ijms20153715. Int J Mol Sci. 2019. PMID: 31366013 Free PMC article. Review.
Cited by
-
Natural Killer Cell Evasion Is Essential for Infection by Rhesus Cytomegalovirus.PLoS Pathog. 2016 Aug 31;12(8):e1005868. doi: 10.1371/journal.ppat.1005868. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27580123 Free PMC article.
-
Immune memory redefined: characterizing the longevity of natural killer cells.Immunol Rev. 2010 Jul;236:83-94. doi: 10.1111/j.1600-065X.2010.00900.x. Immunol Rev. 2010. PMID: 20636810 Free PMC article. Review.
-
Virus encoded MHC-like decoys diversify the inhibitory KIR repertoire.PLoS Comput Biol. 2013;9(10):e1003264. doi: 10.1371/journal.pcbi.1003264. Epub 2013 Oct 10. PLoS Comput Biol. 2013. PMID: 24130473 Free PMC article.
-
Killer Immunoglobulin-like Receptors (KIR) haplogroups A and B track with Natural Killer Cells and Cytokine Profile in Aged Subjects: Observations from Octo/Nonagenarians in the Belfast Elderly Longitudinal Free-living Aging STudy (BELFAST).Immun Ageing. 2013 Aug 19;10(1):35. doi: 10.1186/1742-4933-10-35. Immun Ageing. 2013. PMID: 23957956 Free PMC article.
-
CD1d Expression and Invariant NKT Cell Responses in Herpesvirus Infections.Front Immunol. 2015 Jun 25;6:312. doi: 10.3389/fimmu.2015.00312. eCollection 2015. Front Immunol. 2015. PMID: 26161082 Free PMC article. Review.
References
-
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
-
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. - PubMed
-
- Gilbert SC, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood BM, Whittle HC, Hill AV. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–1177. - PubMed
-
- Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. - PubMed
-
- Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. - PubMed

