Achieving cholesterol targets by individualizing starting doses of statin according to baseline low-density lipoprotein cholesterol and coronary artery disease risk category: the CANadians Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (CanACTFAST) study
- PMID: 20151053
- PMCID: PMC2851387
- DOI: 10.1016/s0828-282x(10)70003-9
Achieving cholesterol targets by individualizing starting doses of statin according to baseline low-density lipoprotein cholesterol and coronary artery disease risk category: the CANadians Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (CanACTFAST) study
Abstract
Background: Despite an increasing body of evidence on the benefit of lowering elevated levels of low-density lipoprotein cholesterol (LDL-C), there is still considerable concern that patients are not achieving target LDL-C levels.
Objective: The CANadians Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (CanACTFAST) trial tested whether an algorithm-based statin dosing approach would enable patients to achieve LDL-C and total cholesterol/high-density lipoprotein cholesterol (TC/HDL-C) ratio targets quickly.
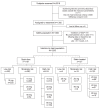
Methods: Subjects requiring statin therapy, but with an LDL-C level of 5.7 mmol/L or lower, and triglycerides of 6.8 mmol/L or lower at screening participated in the 12-week study, which had two open-label, six-week phases: a treatment period during which patients received 10 mg, 20 mg, 40 mg or 80 mg of atorvastatin based on an algorithm incorporating baseline LDL-C value and cardiovascular risk; and patients who achieved both LDL-C and TC/HDL-C ratio targets at six weeks continued on the same atorvastatin dose. Patients who did not achieve both targets received dose uptitration using a single-step titration regimen. The primary efficacy outcome was the proportion of patients achieving target LDL-C levels after 12 weeks.
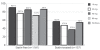
Results: Of 2016 subjects screened at 88 Canadian sites, 1258 were assigned to a study drug (1101 were statin-free and 157 were statin-treated at baseline). The proportion of subjects who achieved LDL-C targets after 12 weeks of treatment was 86% (95% CI 84% to 88%) for statin-free patients and 54% (95% CI 46% to 61%) for statin-treated patients. Overall, 1003 subjects (80%; 95% CI 78% to 82%) achieved both lipid targets.
Conclusions: Algorithm-based statin dosing enables patients to achieve LDL-C and TC/HDL-C ratio targets quickly, with either no titration or a single titration.
HISTORIQUE :: Malgré un ensemble de preuves croissant sur les bienfaits d’abaisser les taux élevés de cholestérol à lipoprotéines de basse densité (C-LDL), on s’inquiète encore beaucoup du fait que les patients n’atteignent pas les taux de C-LDL ciblés.
OBJECTIF :: L’étude CanACTFAST sur l’atteinte des cibles de cholestérol par les Canadiens au moyen du titrage stratifié d’atorvastatine était conçue pour évaluer si une posologie de statines calculée à l’aide d’un algorithme permettrait aux patients d’atteindre rapidement les cibles de C-LDL et de ratio entre le cholestérol total et le cholestérol à lipoprotéines de haute densité (CT/C-HDL).
MÉTHODOLOGIE :: Les sujets qui avaient besoin d’une thérapie aux statines, mais dont le taux de C-LDL était de 5,7 mmol/L ou moins et le taux de triglycérides, de 6,8 mmol/L ou moins au moment du dépistage, ont participé à l’étude de 12 semaines, pourvue de deux phases ouvertes de six semaines : une période de traitement pendant laquelle les patients recevaient 10 mg, 20 mg, 40 mg ou 80 mg d’atorvastatine selon un algorithme intégrant la valeur de C-LDL de départ et le risque cardiovasculaire; les patients qui avaient atteint les cibles de C-LDL et de ratio CT/C-HDL au bout de six semaines maintenaient la même dose d’atorvastatine. Les patients qui n’atteignaient pas les deux cibles ont reçu un surtitrage de la dose au moyen d’une posologie par simple titrage. La proportion de patients atteignant les taux de C-LDL au bout de 12 semaines était l’issue d’efficacité primaire.
RÉSULTATS :: Des 2 016 sujets ayant fait l’objet d’un dépistage dans 88 centres canadiens, 1 258 ont reçu à un médicament à l’étude (1 101 ne prenaient pas de statines et 157 recevaient un traitement aux statines dès le départ). Ainsi, 86 % des sujets ne prenant pas de statines ont atteint les cibles de C-LDL au bout de 12 semaines de traitement (95 % IC 84 % à 88 %) par rapport à 54 % de ceux en recevant (95 % IC 46% à 61 %). Dans l’ensemble, 1 003 sujets (80 %; 95 % IC 78 % à 82 %) ont atteint les deux cibles lipidiques.
CONCLUSIONS :: Une posologie de statines calculée au moyen d’un algorithme permet aux patients d’atteindre rapidement les cibles de C-LDL et de ratio CT/C-HDL, sans titrage ou au moyen d’un seul titrage.
Trial registration: ClinicalTrials.gov NCT00150371.
Figures
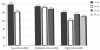
References
-
- Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. - PubMed
-
- Sacks FM, Pfeffer MA, Moye LA. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. - PubMed
-
- Baignet C, Keech A, Kearney PM, et al. for the Cholesterol Treatment Trialists (CTT) Collaboration Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet. 2005;366:1267–78. - PubMed
-
- Fodor JG, McPherson R, Dafoe WA, Grenville A. Investigation and treatment of hypercholesterolemia and other dyslipidemias in Canadian primary care practice. CVD Prevention. 1998;1:225–30.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

