Stability of 5-fluoro-2'-deoxycytidine and tetrahydrouridine in combination
- PMID: 20151336
- PMCID: PMC2850501
- DOI: 10.1208/s12249-010-9383-2
Stability of 5-fluoro-2'-deoxycytidine and tetrahydrouridine in combination
Abstract
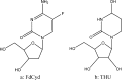
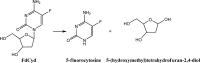
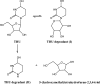
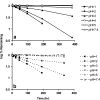
In vivo, the DNA methyltransferase inhibitor, 5-fluoro-2'-deoxycytidine (FdCyd, NSC-48006), is rapidly converted to its unwanted metabolites. Tetrahydrouridine (THU, NSC-112907), a cytidine deaminase inhibitor can block the first metabolic step in FdCyd catabolism. Clinical studies have shown that co-administration with THU can inhibit the metabolism of FdCyd. The National Cancer Institute is particularly interested in a 1:5 FdCyd/THU formulation. The purpose of this study was to investigate the in vitro pH stability of FdCyd and THU individually and in combination. A stability-indicating high-performance liquid chromatography method for the quantification of both compounds and their degradants was developed using a ZIC(R)-HILIC column. The effect of THU and FdCyd on the in vitro degradation of each other was studied as a function of pH from 1.0 to 7.4 in aqueous solutions at 37 degrees C. The degradation of FdCyd appears to be first-order and acid-catalyzed. THU equilibrates with at least one of its degradants. The combination of FdCyd and THU in solution does not affect the stability of either compound. The stability and compatibility of FdCyd and THU in the solid state at increased relative humidity and at various temperatures are also evaluated.
Figures




Similar articles
-
Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU).Cancer Chemother Pharmacol. 2008 Jul;62(2):363-8. doi: 10.1007/s00280-007-0603-8. Epub 2007 Sep 26. Cancer Chemother Pharmacol. 2008. PMID: 17899082 Clinical Trial.
-
Pharmacokinetics, metabolism, and oral bioavailability of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine in mice.Clin Cancer Res. 2006 Dec 15;12(24):7483-91. doi: 10.1158/1078-0432.CCR-06-1250. Epub 2006 Nov 30. Clin Cancer Res. 2006. PMID: 17138702
-
Oral and intravenous pharmacokinetics of 5-fluoro-2'-deoxycytidine and THU in cynomolgus monkeys and humans.Cancer Chemother Pharmacol. 2015 Oct;76(4):803-11. doi: 10.1007/s00280-015-2857-x. Epub 2015 Sep 1. Cancer Chemother Pharmacol. 2015. PMID: 26321472 Free PMC article. Clinical Trial.
-
Epimer interconversion, isomerization, and hydrolysis of tetrahydrouridine: implications for cytidine deaminase inhibition.J Pharm Sci. 2003 Oct;92(10):2027-39. doi: 10.1002/jps.10447. J Pharm Sci. 2003. PMID: 14502542
-
Deoxyuridine analog nucleotides in deoxycytidine analog treatment: secondary active metabolites?Fundam Clin Pharmacol. 2011 Apr;25(2):172-85. doi: 10.1111/j.1472-8206.2010.00823.x. Fundam Clin Pharmacol. 2011. PMID: 20199587 Review.
Cited by
-
Preclinical studies of 5-fluoro-2'-deoxycytidine and tetrahydrouridine in pediatric brain tumors.J Neurooncol. 2016 Jan;126(2):225-34. doi: 10.1007/s11060-015-1965-0. Epub 2015 Oct 30. J Neurooncol. 2016. PMID: 26518542 Free PMC article.
-
Digging deep into "dirty" drugs - modulation of the methylation machinery.Drug Metab Rev. 2015 May;47(2):252-79. doi: 10.3109/03602532.2014.995379. Epub 2015 Jan 8. Drug Metab Rev. 2015. PMID: 25566693 Free PMC article. Review.
-
The Emerging Role of Cytidine Deaminase in Human Diseases: A New Opportunity for Therapy?Mol Ther. 2020 Feb 5;28(2):357-366. doi: 10.1016/j.ymthe.2019.11.026. Epub 2019 Dec 6. Mol Ther. 2020. PMID: 31870623 Free PMC article. Review.
-
Tetrahydrouridine inhibits cell proliferation through cell cycle regulation regardless of cytidine deaminase expression levels.PLoS One. 2012;7(5):e37424. doi: 10.1371/journal.pone.0037424. Epub 2012 May 16. PLoS One. 2012. PMID: 22616006 Free PMC article.
-
DNA methylation profiles in cancer diagnosis and therapeutics.Clin Exp Med. 2018 Feb;18(1):1-14. doi: 10.1007/s10238-017-0467-0. Epub 2017 Jul 27. Clin Exp Med. 2018. PMID: 28752221 Review.
References
-
- Beumer JH, Parise RA, Newman EM, Doroshow JH, Synold TW, Lenz H-J, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU) Cancer Chemother Pharmacol. 2008;62:363–368. doi: 10.1007/s00280-007-0603-8. - DOI - PubMed
-
- Boothman DA, Briggle TV, Greer S. Tumor-selective metabolism of 5-fluoro-2′-deoxycytidine coadministered with tetrahydrouridine compared to 5-fluorouracil in mice bearing Lewis lung carcinoma. Cancer Res. 1987;47:2354–2362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

