Ciprofloxacin as ocular liposomal hydrogel
- PMID: 20151337
- PMCID: PMC2850497
- DOI: 10.1208/s12249-009-9373-4
Ciprofloxacin as ocular liposomal hydrogel
Abstract
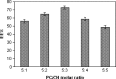
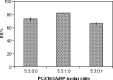
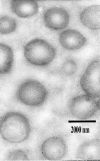
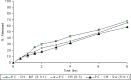
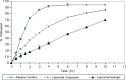
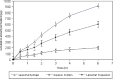
The purpose of this study was to prepare and characterize an ocular effective prolonged-release liposomal hydrogel formulation containing ciprofloxacin. Reverse-phase evaporation was used for preparation of liposomes consisting of soybean phosphatidylcholine (PC) and cholesterol (CH). The effect of PC/CH molar ratio on the percentage drug encapsulation was investigated. The effect of additives such as stearylamine (SA) or dicetyl phosphate (DP) as positive and negative charge inducers, respectively, were studied. Morphology, mean size, encapsulation efficiency, and in vitro release of ciprofloxacin from liposomes were evaluated. For hydrogel preparation, Carbopol 940 was applied. In vitro transcorneal permeation through excised albino rabbit cornea was also determined. Optimal encapsulation efficiency of 73.04 +/- 3.06% was obtained from liposomes formulated with PC/CH at molar ratio of 5:3 and by increasing CH content above this limit, the encapsulation decreased. Positively charged liposomes showed superior entrapment efficiency (82.01 +/- 0.52) over the negatively charged and the neutral liposomes. Hydrogel containing liposomes with lipid content PC, CH, and SA in molar ratio 5:3:1, respectively, showed the best release and transcorneal permeation with the percentage permeation of 30.6%. These results suggest that the degree of encapsulation of ciprofloxacin into liposomes and prolonged in vitro release depend on composition of the vesicles. In addition, the polymer hydrogel used in preparation ensure steady and prolonged transcorneal permeation. In conclusion, ciprofloxacin liposomal hydrogel is a suitable delivery system for improving the ocular bioavailability of ciprofloxacin.
Figures
Similar articles
-
Optimization of gatifloxacin liposomal hydrogel for enhanced transcorneal permeation.J Liposome Res. 2010 Mar;20(1):31-7. doi: 10.3109/08982100903030255. J Liposome Res. 2010. PMID: 19545203
-
Preparation and evaluation of thermosensitive liposomal hydrogel for enhanced transcorneal permeation of ofloxacin.AAPS PharmSciTech. 2009;10(4):1336-42. doi: 10.1208/s12249-009-9335-x. Epub 2009 Nov 10. AAPS PharmSciTech. 2009. PMID: 19902361 Free PMC article.
-
Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes.Pharm Dev Technol. 2011 Feb;16(1):44-56. doi: 10.3109/10837450903479988. Epub 2009 Dec 21. Pharm Dev Technol. 2011. PMID: 20025433
-
Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation.Int J Pharm. 2006 Feb 3;308(1-2):140-8. doi: 10.1016/j.ijpharm.2005.11.006. Epub 2005 Dec 13. Int J Pharm. 2006. PMID: 16356669
-
Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies.AAPS PharmSciTech. 2007 Jan 5;8(1):1. doi: 10.1208/pt0801001. AAPS PharmSciTech. 2007. PMID: 17408209 Free PMC article.
Cited by
-
Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery.Langmuir. 2022 Nov 15;38(45):13870-13879. doi: 10.1021/acs.langmuir.2c02086. Epub 2022 Nov 3. Langmuir. 2022. PMID: 36327096 Free PMC article.
-
An Investigation into Some Effective Factors on Encapsulation Efficiency of Alpha-Tocopherol in MLVs and the Release Profile from the Corresponding Liposomal Gel.Iran J Pharm Res. 2013 Winter;12(Suppl):21-30. Iran J Pharm Res. 2013. PMID: 24250668 Free PMC article.
-
Recent applications of liposomes in ophthalmic drug delivery.J Drug Deliv. 2011;2011:863734. doi: 10.1155/2011/863734. Epub 2011 Mar 1. J Drug Deliv. 2011. PMID: 21490757 Free PMC article.
-
Nanostructured Lipid Carrier-loaded In Situ Gel for Ophthalmic Drug Delivery: Preparation and In Vitro Characterization Studies.Pharm Nanotechnol. 2025;13(1):171-183. doi: 10.2174/0122117385266639231029192409. Pharm Nanotechnol. 2025. PMID: 38213174
-
Ciprofloxacin Loaded Nanostructured Lipid Carriers Incorporated into In-Situ Gels to Improve Management of Bacterial Endophthalmitis.Pharmaceutics. 2020 Jun 19;12(6):572. doi: 10.3390/pharmaceutics12060572. Pharmaceutics. 2020. PMID: 32575524 Free PMC article.
References
-
- Le Bourlais CL, Acar L, Zia H, Sado PA, Needham T, et al. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res. 1998;17:35–58. - PubMed
-
- Adenis JP, Colin J, Verin P, Saint-Blancat P, Malet F. Ciprofloxacin ophthalmic solution versus rifamycin ophthalmic solution for the treatment of conjunctivitis and blepharitis. Eur J Ophthalmol. 1995;5:82–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

