Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice
- PMID: 20151365
- PMCID: PMC2867111
- DOI: 10.1002/cne.22282
Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice
Abstract
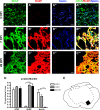
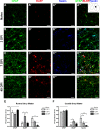
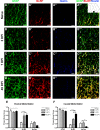
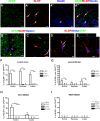
Astrocytes and their precursors respond to spinal cord injury (SCI) by proliferating, migrating, and altering phenotype. This contributes to glial scar formation at the lesion border and gliosis in spared gray and white matter. The present study was undertaken to evaluate astrocyte changes over time and determine when and where interventions might be targeted to alter the astrocyte response. Bromodeoxyuridine (BrdU) was administered to mice 3 days after SCI, and cells expressing BrdU and the astrocyte marker, glial fibrillary acidic protein (GFAP), were counted at 3, 7, and 49 days post-injury (DPI). BrdU-labeled cells accumulated at the lesion border by 7 DPI and approximately half of these expressed GFAP. In spared white matter, the total number of BrdU+ cells decreased, while the percentage of BrdU+ cells expressing GFAP increased at 49 DPI. Phenotypic changes were examined using the progenitor marker nestin, the radial glial marker, brain lipid binding protein (BLBP), and GFAP. Nestin was upregulated by 3 DPI and declined between 7 and 49 DPI in all regions, and GFAP increased and remained above naïve levels at all timepoints. BLBP increased early and remained high along the lesion border and spared white matter, but was expressed transiently by cells lining the central canal and in a unique population of small cells found within the lesion and in gray matter rostral and caudal to the border. The results demonstrate that the astrocyte response to SCI is regionally heterogeneous, and suggests astrocyte populations that could be targeted by interventions.
(c) 2009 Wiley-Liss, Inc.
Figures





References
-
- Abe Y, Yamamoto T, Sugiyama Y, Watanabe T, Saito N, Kayama H, Kumagai T. Apoptotic cells associated with Wallerian degeneration after experimental spinal cord injury: A possible mechanism of oligodendoglial death. J Neurotrauma. 1999;16:945–952. - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. GLIA. 2005;50:187–197. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
-
- Beck T, Lutz B, Theole U, Wree A. Assessing chronic brain damage by quantification of regional volumes in postischemic rat brains. Brain Res. 1993;605:280–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

