Adsorption of egg phosphatidylcholine to an air/water and triolein/water bubble interface: use of the 2-dimensional phase rule to estimate the surface composition of a phospholipid/triolein/water surface as a function of surface pressure
- PMID: 20151713
- PMCID: PMC2851238
- DOI: 10.1021/jp908730t
Adsorption of egg phosphatidylcholine to an air/water and triolein/water bubble interface: use of the 2-dimensional phase rule to estimate the surface composition of a phospholipid/triolein/water surface as a function of surface pressure
Abstract
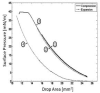
Phospholipid monolayers play a critical role in the structure and stabilization of biological interfaces, including all membranes, the alveoli of the lungs, fat droplets in adipose tissue, and lipoproteins. The behavior of phospholipids in bilayers and at an air-water interface is well understood. However, the study of phospholipids at oil-water interfaces is limited due to technical challenges. In this study, egg phosphatidylcholine (EPC) was deposited from small unilamellar vesicles onto a bubble of either air or triolein (TO) formed in a low-salt buffer. The surface tension (gamma) was measured using a drop tensiometer. We observed that EPC binds irreversibly to both interfaces and at equilibrium exerts approximately 12 and 15 mN/m of pressure (Pi) at an air and TO interface, respectively. After EPC was bound to the interface, the unbound EPC was washed out of the cuvette, and the surface was compressed to study the Pi/area relationship. To determine the surface concentration (Gamma), which cannot be measured directly, compression isotherms from a Langmuir trough and drop tensiometer were compared. The air-water interfaces had identical characteristics using both techniques; thus, Gamma on the bubble can be determined by overlaying the two isotherms. Both TO and EPC are surface-active, so in a mixed TO/EPC monolayer, both molecules will be exposed to water. Since TO is less surface-active than EPC, as Pi increases, the TO is progressively ejected. To understand the Pi/area isotherm of EPC on a TO bubble, a variety of TO-EPC mixtures were spread at the air-water interface. The isotherms show an abrupt break in the curve caused by the ejection of TO from the monolayer into a new bulk phase. By overlaying the compression isotherm above the ejection point with a TO bubble compression isotherm, Gamma can be estimated. This allows determination of Gamma of EPC on a TO bubble as a function of Pi.
Figures








Similar articles
-
Apolipoprotein C-I binds more strongly to phospholipid/triolein/water than triolein/water interfaces: a possible model for inhibiting cholesterol ester transfer protein activity and triacylglycerol-rich lipoprotein uptake.Biochemistry. 2012 Feb 14;51(6):1238-48. doi: 10.1021/bi2015212. Epub 2012 Feb 2. Biochemistry. 2012. PMID: 22264166 Free PMC article.
-
Determining the Dependence of Interfacial Tension on Molecular Area for Phospholipid Monolayers Formed at Silicone Oil-Water and Tricaprylin-Water Interfaces by Vesicle Fusion.Langmuir. 2021 Jun 22;37(24):7527-7535. doi: 10.1021/acs.langmuir.1c01021. Epub 2021 Jun 11. Langmuir. 2021. PMID: 34115510
-
Eutectic mixed monolayers in equilibrium with phospholipid-bilayers and triolein-liquid phase.Biophys J. 1993 Jun;64(6):1760-5. doi: 10.1016/S0006-3495(93)81547-9. Biophys J. 1993. PMID: 8369406 Free PMC article.
-
C-terminus of apolipoprotein A-I removes phospholipids from a triolein/phospholipids/water interface, but the N-terminus does not: a possible mechanism for nascent HDL assembly.Biophys J. 2011 Jul 20;101(2):353-61. doi: 10.1016/j.bpj.2011.03.055. Biophys J. 2011. PMID: 21767487 Free PMC article.
-
Influence of alkane and perfluorocarbon vapors on adsorbed surface layers and spread insoluble monolayers of surfactants, proteins and lipids.Adv Colloid Interface Sci. 2017 Jun;244:100-112. doi: 10.1016/j.cis.2015.11.004. Epub 2015 Dec 2. Adv Colloid Interface Sci. 2017. PMID: 26656422 Review.
Cited by
-
Surface pressure-dependent conformation change of apolipoprotein-derived amphipathic α-helices.J Lipid Res. 2013 Jun;54(6):1578-1588. doi: 10.1194/jlr.M034462. Epub 2013 Mar 25. J Lipid Res. 2013. PMID: 23528259 Free PMC article.
-
A Pressure-dependent Model for the Regulation of Lipoprotein Lipase by Apolipoprotein C-II.J Biol Chem. 2015 Jul 17;290(29):18029-18044. doi: 10.1074/jbc.M114.629865. Epub 2015 May 29. J Biol Chem. 2015. PMID: 26026161 Free PMC article.
-
Interfacial tension and surface pressure of high density lipoprotein, low density lipoprotein, and related lipid droplets.Biophys J. 2012 Sep 19;103(6):1236-44. doi: 10.1016/j.bpj.2012.08.023. Biophys J. 2012. PMID: 22995496 Free PMC article.
-
Apolipoprotein C-I binds more strongly to phospholipid/triolein/water than triolein/water interfaces: a possible model for inhibiting cholesterol ester transfer protein activity and triacylglycerol-rich lipoprotein uptake.Biochemistry. 2012 Feb 14;51(6):1238-48. doi: 10.1021/bi2015212. Epub 2012 Feb 2. Biochemistry. 2012. PMID: 22264166 Free PMC article.
-
Phospholipids at the interface: current trends and challenges.Int J Mol Sci. 2013 Jun 3;14(6):11767-94. doi: 10.3390/ijms140611767. Int J Mol Sci. 2013. PMID: 23736688 Free PMC article. Review.
References
-
- Mahley RW, Innerarity TL, Rall SC, Weisgraber KH. Journal of Lipid Research. 1984;25:1277. - PubMed
-
- Wustneck N, Wustneck R, Fainerman VB, Miller R, Pison U. Colloids and Surfaces B-Biointerfaces. 2001;21:191. - PubMed
-
- Brasaemle DL. Journal of Lipid Research. 2007;48:2547. - PubMed
-
- Small DM. The physical chemistry of lipids : from alkanes to phospholipids. Plenum Press; New York: 1986.
-
- Mohwald H. Phospholipid Monolayers. In: Lipowsky R, editor. Handbook of Biological Physics. Vol. 1. Elsevier Science B.V.; 1995. p. 187.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

