3-Methylcholanthrene-induced transforming growth factor-beta-producing carcinomas, but not sarcomas, are refractory to regulatory T cell-depletion therapy
- PMID: 20151983
- PMCID: PMC11159729
- DOI: 10.1111/j.1349-7006.2009.01469.x
3-Methylcholanthrene-induced transforming growth factor-beta-producing carcinomas, but not sarcomas, are refractory to regulatory T cell-depletion therapy
Abstract
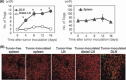
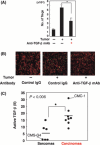
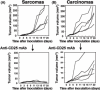
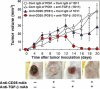
Regulatory T cells (Tregs) are major immunosuppressors in tumor-bearing hosts. Although Treg-depletion therapy has been shown to induce a complete cure in tumor-bearing mice, this treatment is not always successful. Using 3-methylcholanthrene-induced primary mouse tumors, we examined the distinct regulation of Treg-mediated immunosuppression between carcinomas and sarcomas. We showed that the number of Tregs was greatly increased in squamous cell carcinoma (SCC)-bearing mice compared with sarcoma-bearing mice. This appeared to be because SCC produced higher levels of active transforming growth factor (TGF)-beta, which is essential for inducing Tregs, compared with sarcoma. Moreover, SCC, but not sarcomas, were refractory to Treg-depletion therapy by treatment with anti-CD25 mAb. The refractoriness of SCC against Treg-depletion therapy was due to the rapid recovery of Tregs in SCC-bearing mice compared with sarcoma-bearing mice. However, combination treatment of anti-TGF-beta mAb with anti-CD25 mAb caused a significant reduction in Treg recovery and induced a complete cure in SCC-bearing mice. Thus, we showed the refractoriness of mouse carcinoma against Treg-depletion therapy using anti-CD25 mAb treatment. We also proposed a novel Treg-blocking combination therapy using anti-CD25 mAb and anti-TGF-beta mAb to induce a complete cure of tumor-bearing hosts.
Figures


Similar articles
-
Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells.J Transl Med. 2019 Jul 9;17(1):219. doi: 10.1186/s12967-019-1967-3. J Transl Med. 2019. PMID: 31288845 Free PMC article.
-
CD25+ T cell depletion impairs murine squamous cell carcinoma development via modulation of antitumor immune responses.Carcinogenesis. 2012 Apr;33(4):902-9. doi: 10.1093/carcin/bgs103. Epub 2012 Feb 16. Carcinogenesis. 2012. PMID: 22345289
-
Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice.J Reprod Immunol. 2010 Jun;85(2):121-9. doi: 10.1016/j.jri.2010.02.006. Epub 2010 May 2. J Reprod Immunol. 2010. PMID: 20439117
-
IL-7 Mediated Homeostatic Expansion of Human CD4+CD25+FOXP3+ Regulatory T Cells After Depletion With Anti-CD25 Monoclonal Antibody.Transplantation. 2016 Sep;100(9):1853-61. doi: 10.1097/TP.0000000000001276. Transplantation. 2016. PMID: 27306531
-
TGF-beta and tumors--an ill-fated alliance.Curr Opin Immunol. 2008 Apr;20(2):234-40. doi: 10.1016/j.coi.2008.04.003. Epub 2008 May 15. Curr Opin Immunol. 2008. PMID: 18486463 Free PMC article. Review.
Cited by
-
IL-11 induces differentiation of myeloid-derived suppressor cells through activation of STAT3 signalling pathway.Sci Rep. 2015 Sep 1;5:13650. doi: 10.1038/srep13650. Sci Rep. 2015. PMID: 28781374 Free PMC article.
-
Cytoskeletal protein Flightless I inhibits apoptosis, enhances tumor cell invasion and promotes cutaneous squamous cell carcinoma progression.Oncotarget. 2015 Nov 3;6(34):36426-40. doi: 10.18632/oncotarget.5536. Oncotarget. 2015. PMID: 26497552 Free PMC article.
-
Basic principles of tumor-associated regulatory T cell biology.Trends Immunol. 2013 Jan;34(1):33-40. doi: 10.1016/j.it.2012.08.005. Epub 2012 Sep 19. Trends Immunol. 2013. PMID: 22999714 Free PMC article. Review.
-
Enhancement of antitumor effect by peptide vaccine therapy in combination with anti-CD4 antibody: Study in a murine model.Biochem Biophys Rep. 2016 Feb 19;5:482-491. doi: 10.1016/j.bbrep.2016.02.010. eCollection 2016 Mar. Biochem Biophys Rep. 2016. PMID: 28955856 Free PMC article.
References
-
- Colombo MP, Piconese S. Regulatory T‐cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007; 7: 880–7. - PubMed

