BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs
- PMID: 20152028
- PMCID: PMC2829471
- DOI: 10.1186/1471-213X-10-15
BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs
Abstract
Background: Axolotls have the unique ability, among vertebrates, to perfectly regenerate complex body parts, such as limbs, after amputation. In addition, axolotls pattern developing and regenerating autopods from the anterior to posterior axis instead of posterior to anterior like all tetrapods studied to date. Sonic hedgehog is important in establishing this anterior-posterior axis of limbs in all tetrapods including axolotls. Interestingly, its expression is conserved (to the posterior side of limb buds and blastemas) in axolotl limbs as in other tetrapods. It has been suggested that BMP-2 may be the secondary mediator of sonic hedgehog, although there is mounting evidence to the contrary in mice. Since BMP-2 expression is on the anterior portion of developing and regenerating limbs prior to digit patterning, opposite to the expression of sonic hedgehog, we examined whether BMP-2 expression was dependent on sonic hedgehog signaling and whether it affects patterning of the autopod during regeneration.
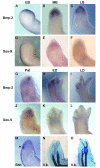
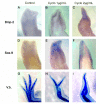
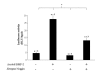
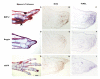
Results: The expression of BMP-2 and SOX-9 in developing and regenerating axolotl limbs corresponded to the first digits forming in the anterior portion of the autopods. The inhibition of sonic hedgehog signaling with cyclopamine caused hypomorphic limbs (during development and regeneration) but did not affect the expression of BMP-2 and SOX-9. Overexpression of BMP-2 in regenerating limbs caused a loss of digits. Overexpression of Noggin (BMP inhibitor) in regenerating limbs also resulted in a loss of digits. Histological analysis indicated that the loss due to BMP-2 overexpression was the result of increased cell condensation and apoptosis while the loss caused by Noggin was due to a decrease in cell division.
Conclusion: The expression of BMP-2 and its target SOX-9 was independent of sonic hedgehog signaling in developing and regenerating limbs. Their expression correlated with chondrogenesis and the appearance of skeletal elements has described in other tetrapods. Overexpression of BMP-2 did not cause the formation of extra digits, which is consistent with the hypothesis that it is not the secondary signal of sonic hedgehog. However, it did cause the formation of hypomorphic limbs as a result of increased cellular condensation and apoptosis. Taken together, these results suggest that BMP-2 does not have a direct role in patterning regenerating limbs but may be important to trigger condensation prior to ossification and to mediate apoptosis.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

