Protein engineering of conger eel galectins by tracing of molecular evolution using probable ancestral mutants
- PMID: 20152053
- PMCID: PMC2843614
- DOI: 10.1186/1471-2148-10-43
Protein engineering of conger eel galectins by tracing of molecular evolution using probable ancestral mutants
Abstract
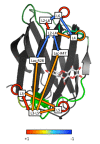
Background: Conger eel galectins, congerin I (ConI) and congerin II (ConII), show the different molecular characteristics resulting from accelerating evolution. We recently reconstructed a probable ancestral form of congerins, Con-anc. It showed properties similar to those of ConII in terms of thermostability and carbohydrate recognition specificity, although it shares a higher sequence similarity with ConI than ConII.
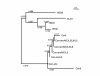
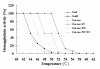
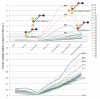
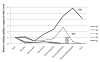
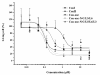
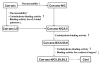
Results: In this study, we have focused on the different amino acid residues between Con-anc and ConI, and have performed the protein engineering of Con-anc through site-directed mutagenesis, followed by the molecular evolution analysis of the mutants. This approach revealed the functional importance of loop structures of congerins: (1) N- and C-terminal and loop 5 regions that are involved in conferring a high thermostability to ConI; (2) loops 3, 5, and 6 that are responsible for stronger binding of ConI to most sugars; and (3) loops 5 and 6, and Thr38 residue in loop 3 contribute the specificity of ConI toward lacto-N-fucopentaose-containing sugars.
Conclusions: Thus, this methodology, with tracing of the molecular evolution using ancestral mutants, is a powerful tool for the analysis of not only the molecular evolutionary process, but also the structural elements of a protein responsible for its various functions.
Figures

Similar articles
-
Allosteric regulation of the carbohydrate-binding ability of a novel conger eel galectin by D-mannoside.J Biol Chem. 2012 Sep 7;287(37):31061-72. doi: 10.1074/jbc.M112.346213. Epub 2012 Jul 18. J Biol Chem. 2012. PMID: 22810239 Free PMC article.
-
Reconstruction of a probable ancestral form of conger eel galectins revealed their rapid adaptive evolution process for specific carbohydrate recognition.Mol Biol Evol. 2007 Nov;24(11):2504-14. doi: 10.1093/molbev/msm185. Epub 2007 Sep 6. Mol Biol Evol. 2007. PMID: 17827170
-
Functional and structural characterization of multiple galectins from the skin mucus of conger eel, Conger myriaster.Comp Biochem Physiol B Biochem Mol Biol. 1999 May;123(1):33-45. doi: 10.1016/s0305-0491(99)00037-1. Comp Biochem Physiol B Biochem Mol Biol. 1999. PMID: 10425711
-
The speciation of conger eel galectins by rapid adaptive evolution.Glycoconj J. 2002;19(7-9):451-8. doi: 10.1023/B:GLYC.0000014074.38755.1d. Glycoconj J. 2002. PMID: 14758068 Review.
-
Structure based studies of the adaptive diversification process of congerins.Mol Divers. 2006 Nov;10(4):567-73. doi: 10.1007/s11030-006-9030-8. Mol Divers. 2006. PMID: 16972013 Review.
Cited by
-
Isolation of novel prototype galectins from the marine ball sponge Cinachyrella sp. guided by their modulatory activity on mammalian glutamate-gated ion channels.Glycobiology. 2013 Apr;23(4):412-25. doi: 10.1093/glycob/cws165. Epub 2012 Dec 4. Glycobiology. 2013. PMID: 23213112 Free PMC article.
-
Molecular co-evolution of a protease and its substrate elucidated by analysis of the activity of predicted ancestral hatching enzyme.BMC Evol Biol. 2013 Oct 25;13:231. doi: 10.1186/1471-2148-13-231. BMC Evol Biol. 2013. PMID: 24161109 Free PMC article.
-
Allosteric regulation of the carbohydrate-binding ability of a novel conger eel galectin by D-mannoside.J Biol Chem. 2012 Sep 7;287(37):31061-72. doi: 10.1074/jbc.M112.346213. Epub 2012 Jul 18. J Biol Chem. 2012. PMID: 22810239 Free PMC article.
-
Diversified carbohydrate-binding lectins from marine resources.J Amino Acids. 2011;2011:838914. doi: 10.4061/2011/838914. Epub 2011 Nov 15. J Amino Acids. 2011. PMID: 22312473 Free PMC article.
References
-
- Nei M. Molecular Evolutionary Genetics. Columbia University Press, NY; 1987.
-
- Hartl DL, Clark AG. Principles of Population Genetics. 3. Sinauer Associates, Sunderland, MA; 1997.
-
- Barondes SH, Castronovo V, Cooper DNW, Cummings RD, Drickamer K, Felzi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F-T, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL. Galectins: A family of animal β-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. - DOI - PubMed
-
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

