5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants
- PMID: 20152112
- PMCID: PMC2941196
- DOI: 10.1016/j.neuron.2009.12.003
5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants
Abstract
Most depressed patients don't respond to their first drug treatment, and the reasons for this treatment resistance remain enigmatic. Human studies implicate a polymorphism in the promoter of the serotonin-1A (5-HT(1A)) receptor gene in increased susceptibility to depression and decreased treatment response. Here we develop a new strategy to manipulate 5-HT(1A) autoreceptors in raphe nuclei without affecting 5-HT(1A) heteroreceptors, generating mice with higher (1A-High) or lower (1A-Low) autoreceptor levels. We show that this robustly affects raphe firing rates, but has no effect on either basal forebrain serotonin levels or conflict-anxiety measures. However, compared to 1A-Low mice, 1A-High mice show a blunted physiological response to acute stress, increased behavioral despair, and no behavioral response to antidepressant, modeling patients with the 5-HT(1A) risk allele. Furthermore, reducing 5-HT(1A) autoreceptor levels prior to antidepressant treatment is sufficient to convert nonresponders into responders. These results establish a causal relationship between 5-HT(1A) autoreceptor levels, resilience under stress, and response to antidepressants.
Figures





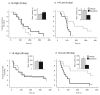

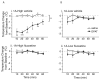

Comment in
-
Altered function of the serotonin 1A autoreceptor and the antidepressant response.Neuron. 2010 Jan 14;65(1):1-2. doi: 10.1016/j.neuron.2009.12.028. Neuron. 2010. PMID: 20152106
Similar articles
-
Acute 5-HT₁A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions.Psychopharmacology (Berl). 2013 Jan;225(1):61-74. doi: 10.1007/s00213-012-2795-9. Epub 2012 Jul 21. Psychopharmacology (Berl). 2013. PMID: 22820867
-
Abrogated Freud-1/Cc2d1a Repression of 5-HT1A Autoreceptors Induces Fluoxetine-Resistant Anxiety/Depression-Like Behavior.J Neurosci. 2017 Dec 6;37(49):11967-11978. doi: 10.1523/JNEUROSCI.1668-17.2017. Epub 2017 Nov 3. J Neurosci. 2017. PMID: 29101244 Free PMC article.
-
Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice.J Biol Chem. 2012 Feb 24;287(9):6615-27. doi: 10.1074/jbc.M111.293027. Epub 2012 Jan 9. J Biol Chem. 2012. PMID: 22232550 Free PMC article.
-
Transcriptional regulation of the 5-HT1A receptor: implications for mental illness.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2402-15. doi: 10.1098/rstb.2011.0376. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826341 Free PMC article. Review.
-
5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function.Psychopharmacology (Berl). 2014 Feb;231(4):623-36. doi: 10.1007/s00213-013-3389-x. Epub 2013 Dec 12. Psychopharmacology (Berl). 2014. PMID: 24337875 Free PMC article. Review.
Cited by
-
The role of 5-HT1A receptors in mediating acute negative effects of antidepressants: implications in pediatric depression.Transl Psychiatry. 2015 May 5;5(5):e563. doi: 10.1038/tp.2015.57. Transl Psychiatry. 2015. PMID: 25942044 Free PMC article.
-
Chronic postnatal chemogenetic activation of forebrain excitatory neurons evokes persistent changes in mood behavior.Elife. 2020 Sep 21;9:e56171. doi: 10.7554/eLife.56171. Elife. 2020. PMID: 32955432 Free PMC article.
-
A comparison of ondansetron in preventing postoperative nausea and vomiting for patients with or without preoperative anxiety with painless egg retrieval: a prospective, randomized, controlled trial.Gland Surg. 2024 Aug 31;13(8):1522-1534. doi: 10.21037/gs-24-175. Epub 2024 Aug 12. Gland Surg. 2024. PMID: 39282027 Free PMC article.
-
Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment.Transl Psychiatry. 2016 Apr 26;6(4):e794. doi: 10.1038/tp.2016.52. Transl Psychiatry. 2016. PMID: 27115125 Free PMC article.
-
Modifying 5-HT1A Receptor Gene Expression as a New Target for Antidepressant Therapy.Front Neurosci. 2010 Jun 17;4:35. doi: 10.3389/fnins.2010.00035. eCollection 2010. Front Neurosci. 2010. PMID: 20661455 Free PMC article.
References
-
- Health, United States. NCfH Statistics, ed. Hyattsville, MD: 2007. 2007 With Chartbook on Trends in the Health of Americans. - PubMed
-
- Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. - PubMed
-
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. - PubMed
-
- Anttila S, Huuhka K, Huuhka M, Rontu R, Hurme M, Leinonen E, Lehtimaki T. Interaction between 5-HT1A and BDNF genotypes increases the risk of treatment-resistant depression. J Neural Transm. 2007;114:1065–1068. - PubMed
-
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

