Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin
- PMID: 20152113
- PMCID: PMC2841506
- DOI: 10.1016/j.neuron.2009.12.007
Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin
Abstract
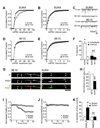
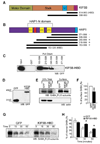
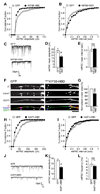
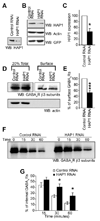
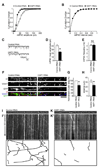
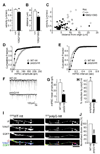
The density of GABA(A) receptors (GABA(A)Rs) at synapses regulates brain excitability, and altered inhibition may contribute to Huntington's disease, which is caused by a polyglutamine repeat in the protein huntingtin. However, the machinery that delivers GABA(A)Rs to synapses is unknown. We demonstrate that GABA(A)Rs are trafficked to synapses by the kinesin family motor protein 5 (KIF5). We identify the adaptor linking the receptors to KIF5 as the huntingtin-associated protein 1 (HAP1). Disrupting the HAP1-KIF5 complex decreases synaptic GABA(A)R number and reduces the amplitude of inhibitory postsynaptic currents. When huntingtin is mutated, as in Huntington's disease, GABA(A)R transport and inhibitory synaptic currents are reduced. Thus, HAP1-KIF5-dependent GABA(A)R trafficking is a fundamental mechanism controlling the strength of synaptic inhibition in the brain. Its disruption by mutant huntingtin may explain some of the defects in brain information processing occurring in Huntington's disease and provides a molecular target for therapeutic approaches.
Figures

Similar articles
-
Disrupted GABAAR trafficking and synaptic inhibition in a mouse model of Huntington's disease.Neurobiol Dis. 2012 May;46(2):497-502. doi: 10.1016/j.nbd.2012.02.015. Epub 2012 Feb 28. Neurobiol Dis. 2012. PMID: 22402331 Free PMC article.
-
Impaired alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking and function by mutant huntingtin.J Biol Chem. 2011 Sep 30;286(39):33719-28. doi: 10.1074/jbc.M111.236521. Epub 2011 Aug 5. J Biol Chem. 2011. PMID: 21832090 Free PMC article.
-
Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking.Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12736-41. doi: 10.1073/pnas.0401860101. Epub 2004 Aug 13. Proc Natl Acad Sci U S A. 2004. PMID: 15310851 Free PMC article.
-
Synaptic dysfunction in Huntington's disease: a new perspective.Cell Mol Life Sci. 2005 Sep;62(17):1901-12. doi: 10.1007/s00018-005-5084-5. Cell Mol Life Sci. 2005. PMID: 15968465 Free PMC article. Review.
-
Huntington's disease: a synaptopathy?Trends Mol Med. 2003 Oct;9(10):414-20. doi: 10.1016/j.molmed.2003.08.006. Trends Mol Med. 2003. PMID: 14557053 Review.
Cited by
-
Deceivingly dynamic: Learning-dependent changes in stathmin and microtubules.Neurobiol Learn Mem. 2015 Oct;124:52-61. doi: 10.1016/j.nlm.2015.07.011. Epub 2015 Jul 26. Neurobiol Learn Mem. 2015. PMID: 26211874 Free PMC article. Review.
-
Altered lysosomal positioning affects lysosomal functions in a cellular model of Huntington's disease.Eur J Neurosci. 2015 Aug;42(3):1941-51. doi: 10.1111/ejn.12957. Epub 2015 Jun 19. Eur J Neurosci. 2015. PMID: 25997742 Free PMC article.
-
Microtubule Motor Transport of Organelles in a Specialized Epithelium: The RPE.Front Cell Dev Biol. 2022 Mar 4;10:852468. doi: 10.3389/fcell.2022.852468. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35309899 Free PMC article. Review.
-
Selective motor activation in organelle transport along axons.Nat Rev Mol Cell Biol. 2022 Nov;23(11):699-714. doi: 10.1038/s41580-022-00491-w. Epub 2022 May 30. Nat Rev Mol Cell Biol. 2022. PMID: 35637414 Review.
-
New approach to capture and characterize synaptic proteome.Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):16154-9. doi: 10.1073/pnas.1401483111. Epub 2014 Oct 28. Proc Natl Acad Sci U S A. 2014. PMID: 25352669 Free PMC article.
References
-
- Arancibia-Carcamo IL, Kittler JT. Regulation of GABA(A) receptor membrane trafficking and synaptic localization. Pharmacol Ther. 2009;123:17–31. - PubMed
-
- Banker G, Goslin K. Culturing nerve cells. 2nd edn. Massachusetts: The MIT Press; 1998.
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

